[English] 日本語
 Yorodumi
Yorodumi- EMDB-29331: Cryo-EM structure of the human TRPV4 in complex with GSK1016790A,... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human TRPV4 in complex with GSK1016790A, TRPV4 focused refinement map | |||||||||
 Map data Map data | main_map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPV4 / RhoA / GSK1016790A / MEMBRANE PROTEIN | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Kwon DH / Lee S-Y / Zhang F | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: TRPV4-Rho GTPase complex structures reveal mechanisms of gating and disease. Authors: Do Hoon Kwon / Feng Zhang / Brett A McCray / Shasha Feng / Meha Kumar / Jeremy M Sullivan / Wonpil Im / Charlotte J Sumner / Seok-Yong Lee /  Abstract: Crosstalk between ion channels and small GTPases is critical during homeostasis and disease, but little is known about the structural underpinnings of these interactions. TRPV4 is a polymodal, ...Crosstalk between ion channels and small GTPases is critical during homeostasis and disease, but little is known about the structural underpinnings of these interactions. TRPV4 is a polymodal, calcium-permeable cation channel that has emerged as a potential therapeutic target in multiple conditions. Gain-of-function mutations also cause hereditary neuromuscular disease. Here, we present cryo-EM structures of human TRPV4 in complex with RhoA in the ligand-free, antagonist-bound closed, and agonist-bound open states. These structures reveal the mechanism of ligand-dependent TRPV4 gating. Channel activation is associated with rigid-body rotation of the intracellular ankyrin repeat domain, but state-dependent interaction with membrane-anchored RhoA constrains this movement. Notably, many residues at the TRPV4-RhoA interface are mutated in disease and perturbing this interface by introducing mutations into either TRPV4 or RhoA increases TRPV4 channel activity. Together, these results suggest that RhoA serves as an auxiliary subunit for TRPV4, regulating TRPV4-mediated calcium homeostasis and disruption of TRPV4-RhoA interactions can lead to TRPV4-related neuromuscular disease. These insights will help facilitate TRPV4 therapeutics development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29331.map.gz emd_29331.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29331-v30.xml emd-29331-v30.xml emd-29331.xml emd-29331.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29331.png emd_29331.png | 80.9 KB | ||
| Others |  emd_29331_half_map_1.map.gz emd_29331_half_map_1.map.gz emd_29331_half_map_2.map.gz emd_29331_half_map_2.map.gz | 59.1 MB 59.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29331 http://ftp.pdbj.org/pub/emdb/structures/EMD-29331 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29331 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29331 | HTTPS FTP |
-Validation report
| Summary document |  emd_29331_validation.pdf.gz emd_29331_validation.pdf.gz | 962 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29331_full_validation.pdf.gz emd_29331_full_validation.pdf.gz | 961.6 KB | Display | |
| Data in XML |  emd_29331_validation.xml.gz emd_29331_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_29331_validation.cif.gz emd_29331_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29331 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29331 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29331 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29331 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29331.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29331.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main_map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
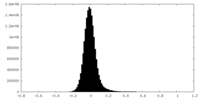
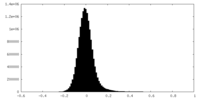
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half B
| File | emd_29331_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
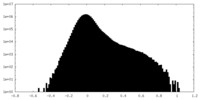
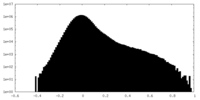
| Density Histograms |
-Half map: half A
| File | emd_29331_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : The complex of human TRPV4 with RhoA
| Entire | Name: The complex of human TRPV4 with RhoA |
|---|---|
| Components |
|
-Supramolecule #1: The complex of human TRPV4 with RhoA
| Supramolecule | Name: The complex of human TRPV4 with RhoA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 450 KDa |
-Macromolecule #1: Human TRPV4
| Macromolecule | Name: Human TRPV4 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MADSSEGPRA GPGEVAELPG DESGTPGGEA FPLSSLANLF EGEDGSLSPS PADASRPAGP GDGRPNLRMK FQGAFRKGVP NPIDLLESTL YESSVVPGPK KAPMDSLFDY GTYRHHSSDN KRWRKKIIEK QPQSPKAPAP QPPPILKVFN RPILFDIVSR GSTADLDGLL ...String: MADSSEGPRA GPGEVAELPG DESGTPGGEA FPLSSLANLF EGEDGSLSPS PADASRPAGP GDGRPNLRMK FQGAFRKGVP NPIDLLESTL YESSVVPGPK KAPMDSLFDY GTYRHHSSDN KRWRKKIIEK QPQSPKAPAP QPPPILKVFN RPILFDIVSR GSTADLDGLL PFLLTHKKRL TDEEFREPST GKTCLPKALL NLSNGRNDTI PVLLDIAERT GNMREFINSP FRDIYYRGQT ALHIAIERRC KHYVELLVAQ GADVHAQARG RFFQPKDEGG YFYFGELPLS LAACTNQPHI VNYLTENPHK KADMRRQDSR GNTVLHALVA IADNTRENTK FVTKMYDLLL LKCARLFPDS NLEAVLNNDG LSPLMMAAKT GKIGIFQHII RREVTDEDTR HLSRKFKDWA YGPVYSSLYD LSSLDTCGEE ASVLEILVYN SKIENRHEML AVEPINELLR DKWRKFGAVS FYINVVSYLC AMVIFTLTAY YQPLEGTPPY PYRTTVDYLR LAGEVITLFT GVLFFFTNIK DLFMKKCPGV NSLFIDGSFQ LLYFIYSVLV IVSAALYLAG IEAYLAVMVF ALVLGWMNAL YFTRGLKLTG TYSIMIQKIL FKDLFRFLLV YLLFMIGYAS ALVSLLNPCA NMKVCNEDQT NCTVPTYPSC RDSETFSTFL LDLFKLTIGM GDLEMLSSTK YPVVFIILLV TYIILTFVLL LNMLIALMGE TVGQVSKESK HIWKLQWATT ILDIERSFPV FLRKAFRSGE MVTVGKSSDG TPDRRWCFRV DEVNWSHWNQ NLGIINEDPG KNETYQYYGF SHTVGRLRRD RWSSVVPRVV ELNKNSNPDE VVVPLDSMGN PRCDGHQQGY PRKWRTDDAP L |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 281.15 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 73349 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)