[English] 日本語
 Yorodumi
Yorodumi- EMDB-27532: Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in com... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 (focused refinement of RBD and ACE2) | |||||||||
 Map data Map data | Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 (focused refinement of RBD and ACE2) | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMetabolism of Angiotensinogen to Angiotensins / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / tryptophan transport / positive regulation of gap junction assembly / regulation of cardiac conduction / peptidyl-dipeptidase activity / maternal process involved in female pregnancy ...Metabolism of Angiotensinogen to Angiotensins / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / tryptophan transport / positive regulation of gap junction assembly / regulation of cardiac conduction / peptidyl-dipeptidase activity / maternal process involved in female pregnancy / carboxypeptidase activity / positive regulation of cardiac muscle contraction / brush border membrane / negative regulation of smooth muscle cell proliferation / negative regulation of ERK1 and ERK2 cascade / cilium / metallopeptidase activity / virus receptor activity / Maturation of spike protein / endopeptidase activity / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / receptor-mediated endocytosis of virus by host cell / membrane fusion / Attachment and Entry / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / receptor ligand activity / host cell surface receptor binding / apical plasma membrane / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / cell surface / proteolysis / extracellular space / identical protein binding / membrane / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
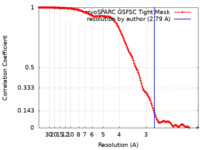
| Method | single particle reconstruction / cryo EM / Resolution: 2.79 Å | |||||||||
 Authors Authors | Zhu X / Saville JW / Mannar D / Berezuk AM / Cholak S / Tuttle KS / Vahdatihassani F / Subramaniam S | |||||||||
| Funding support |  Canada, 2 items Canada, 2 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: Structural analysis of receptor engagement and antigenic drift within the BA.2 spike protein. Authors: James W Saville / Dhiraj Mannar / Xing Zhu / Alison M Berezuk / Spencer Cholak / Katharine S Tuttle / Faezeh Vahdatihassani / Sriram Subramaniam /  Abstract: The BA.2 sub-lineage of the Omicron (B.1.1.529) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant rapidly supplanted the original BA.1 sub-lineage in early 2022. Both lineages ...The BA.2 sub-lineage of the Omicron (B.1.1.529) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant rapidly supplanted the original BA.1 sub-lineage in early 2022. Both lineages threatened the efficacy of vaccine-elicited antibodies and acquired increased binding to several mammalian ACE2 receptors. Cryoelectron microscopy (cryo-EM) analysis of the BA.2 spike (S) glycoprotein in complex with mouse ACE2 (mACE2) identifies BA.1- and BA.2-mutated residues Q493R, N501Y, and Y505H as complementing non-conserved residues between human and mouse ACE2, rationalizing the enhanced S protein-mACE2 interaction for Omicron variants. Cryo-EM structures of the BA.2 S-human ACE2 complex and of the extensively mutated BA.2 amino-terminal domain (NTD) reveal a dramatic reorganization of the highly antigenic N1 loop into a β-strand, providing an explanation for decreased binding of the BA.2 S protein to antibodies isolated from BA.1-convalescent patients. Our analysis reveals structural mechanisms underlying the antigenic drift in the rapidly evolving Omicron variant landscape. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27532.map.gz emd_27532.map.gz | 122.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27532-v30.xml emd-27532-v30.xml emd-27532.xml emd-27532.xml | 18 KB 18 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27532_fsc.xml emd_27532_fsc.xml | 11.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_27532.png emd_27532.png | 75.1 KB | ||
| Others |  emd_27532_half_map_1.map.gz emd_27532_half_map_1.map.gz emd_27532_half_map_2.map.gz emd_27532_half_map_2.map.gz | 226.4 MB 226.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27532 http://ftp.pdbj.org/pub/emdb/structures/EMD-27532 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27532 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27532 | HTTPS FTP |
-Validation report
| Summary document |  emd_27532_validation.pdf.gz emd_27532_validation.pdf.gz | 977.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27532_full_validation.pdf.gz emd_27532_full_validation.pdf.gz | 976.8 KB | Display | |
| Data in XML |  emd_27532_validation.xml.gz emd_27532_validation.xml.gz | 19.3 KB | Display | |
| Data in CIF |  emd_27532_validation.cif.gz emd_27532_validation.cif.gz | 25.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27532 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27532 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27532 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27532 | HTTPS FTP |
-Related structure data
| Related structure data |  8dmaMC  8dm1C  8dm2C  8dm3C  8dm4C  8dm5C  8dm6C  8dm7C  8dm8C  8dm9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27532.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27532.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 (focused refinement of RBD and ACE2) | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein...
| File | emd_27532_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 (focused refinement of RBD and ACE2) | ||||||||||||
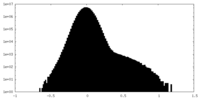
| Projections & Slices |
| ||||||||||||
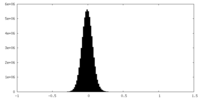
| Density Histograms |
-Half map: Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein...
| File | emd_27532_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 (focused refinement of RBD and ACE2) | ||||||||||||
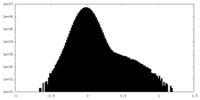
| Projections & Slices |
| ||||||||||||
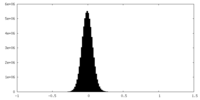
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2
| Entire | Name: SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2
| Supramolecule | Name: SARS-CoV-2 Omicron BA.1 spike protein in complex with mouse ACE2 type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: SARS-CoV-2 Omicron BA.1 spike protein
| Supramolecule | Name: SARS-CoV-2 Omicron BA.1 spike protein / type: complex / ID: 2 / Chimera: Yes / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: mouse ACE2
| Supramolecule | Name: mouse ACE2 / type: complex / ID: 3 / Chimera: Yes / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 142.586156 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHVISG TNGTKRFDNP VLPFNDGVY FASIEKSNII RGWIFGTTLD SKTQSLLIVN NATNVVIKVC EFQFCNDPFL DHKNNKSWME SEFRVYSSAN N CTFEYVSQ ...String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHVISG TNGTKRFDNP VLPFNDGVY FASIEKSNII RGWIFGTTLD SKTQSLLIVN NATNVVIKVC EFQFCNDPFL DHKNNKSWME SEFRVYSSAN N CTFEYVSQ PFLMDLEGKQ GNFKNLREFV FKNIDGYFKI YSKHTPIIVR EPEDLPQGFS ALEPLVDLPI GINITRFQTL LA LHRSYLT PGDSSSGWTA GAAAYYVGYL QPRTFLLKYN ENGTITDAVD CALDPLSETK CTLKSFTVEK GIYQTSNFRV QPT ESIVRF PNITNLCPFD EVFNATRFAS VYAWNRKRIS NCVADYSVLY NLAPFFTFKC YGVSPTKLND LCFTNVYADS FVIR GDEVR QIAPGQTGNI ADYNYKLPDD FTGCVIAWNS NKLDSKVSGN YNYLYRLFRK SNLKPFERDI STEIYQAGNK PCNGV AGFN CYFPLRSYSF RPTYGVGHQP YRVVVLSFEL LHAPATVCGP KKSTNLVKNK CVNFNFNGLK GTGVLTESNK KFLPFQ QFG RDIADTTDAV RDPQTLEILD ITPCSFGGVS VITPGTNTSN QVAVLYQGVN CTEVPVAIHA DQLTPTWRVY STGSNVF QT RAGCLIGAEY VNNSYECDIP IGAGICASYQ TQTKSHGSAS SVASQSIIAY TMSLGAENSV AYSNNSIAIP TNFTISVT T EILPVSMTKT SVDCTMYICG DSTECSNLLL QYGSFCTQLK RALTGIAVEQ DKNTQEVFAQ VKQIYKTPPI KYFGGFNFS QILPDPSKPS KRSPIEDLLF NKVTLADAGF IKQYGDCLGD IAARDLICAQ KFKGLTVLPP LLTDEMIAQY TSALLAGTIT SGWTFGAGP ALQIPFPMQM AYRFNGIGVT QNVLYENQKL IANQFNSAIG KIQDSLSSTP SALGKLQDVV NHNAQALNTL V KQLSSKFG AISSVLNDIF SRLDPPEAEV QIDRLITGRL QSLQTYVTQQ LIRAAEIRAS ANLAATKMSE CVLGQSKRVD FC GKGYHLM SFPQSAPHGV VFLHVTYVPA QEKNFTTAPA ICHDGKAHFP REGVFVSNGT HWFVTQRNFY EPQIITTDNT FVS GNCDVV IGIVNNTVYD PLQPELDSFK EELDKYFKNH TSPDVDLGDI SGINASVVNI QKEIDRLNEV AKNLNESLID LQEL GKYEQ GSGYIPEAPR DGQAYVRKDG EWVLLSTFLG RSLEVLFQGP GHHHHHHHHS AWSHPQFEKG GGSGGGGSGG SAWSH PQFE K |
-Macromolecule #2: Angiotensin-converting enzyme 2
| Macromolecule | Name: Angiotensin-converting enzyme 2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: angiotensin-converting enzyme 2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 71.795523 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSSSSWLLLS LVAVTTAQSL TEENAKTFLN NFNQEAEDLS YQSSLASWNY NTNITEENAQ KMSEAAAKWS AFYEEQSKTA QSFSLQEIQ TPIIKRQLQA LQQSGSSALS ADKNKQLNTI LNTMSTIYST GKVCNPKNPQ ECLLLEPGLD EIMATSTDYN S RLWAWEGW ...String: MSSSSWLLLS LVAVTTAQSL TEENAKTFLN NFNQEAEDLS YQSSLASWNY NTNITEENAQ KMSEAAAKWS AFYEEQSKTA QSFSLQEIQ TPIIKRQLQA LQQSGSSALS ADKNKQLNTI LNTMSTIYST GKVCNPKNPQ ECLLLEPGLD EIMATSTDYN S RLWAWEGW RAEVGKQLRP LYEEYVVLKN EMARANNYND YGDYWRGDYE AEGADGYNYN RNQLIEDVER TFAEIKPLYE HL HAYVRRK LMDTYPSYIS PTGCLPAHLL GDMWGRFWTN LYPLTVPFAQ KPNIDVTDAM MNQGWDAERI FQEAEKFFVS VGL PHMTQG FWANSMLTEP ADGRKVVCHP TAWDLGHGDF RIKMCTKVTM DNFLTAHHEM GHIQYDMAYA RQPFLLRNGA NEGF HEAVG EIMSLSAATP KHLKSIGLLP SDFQEDSETE INFLLKQALT IVGTLPFTYM LEKWRWMVFR GEIPKEQWMK KWWEM KREI VGVVEPLPHD ETYCDPASLF HVSNDYSFIR YYTRTIYQFQ FQEALCQAAK YNGSLHKCDI SNSTEAGQKL LKMLSL GNS EPWTKALENV VGARNMDVKP LLNYFQPLFD WLKEQNRNSF VGWNTEWSPY ADHHHHHH |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z
Z Y
Y X
X