+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24001 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Thermotoga maritima encapsulin shell | |||||||||
 Map data Map data | Final cryo-EM map for the T maritima encapsulin shell | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Encapsulin / Ferritin-like protein / EncFLP / Thermotoga / FMN / Bacterial Nanocompartment / VIRUS LIKE PARTICLE | |||||||||
| Function / homology |  Function and homology information Function and homology informationencapsulin nanocompartment / Hydrolases; Acting on peptide bonds (peptidases) / peptidase activity / iron ion transport / intracellular iron ion homeostasis / proteolysis Similarity search - Function | |||||||||
| Biological species |   Thermotoga maritima (bacteria) Thermotoga maritima (bacteria) | |||||||||
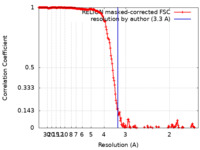
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | LaFrance BJ / Nogales E | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2021 Journal: Sci Rep / Year: 2021Title: The encapsulin from Thermotoga maritima is a flavoprotein with a symmetry matched ferritin-like cargo protein. Authors: Benjamin J LaFrance / Caleb Cassidy-Amstutz / Robert J Nichols / Luke M Oltrogge / Eva Nogales / David F Savage /  Abstract: Bacterial nanocompartments, also known as encapsulins, are an emerging class of protein-based 'organelles' found in bacteria and archaea. Encapsulins are virus-like icosahedral particles comprising a ...Bacterial nanocompartments, also known as encapsulins, are an emerging class of protein-based 'organelles' found in bacteria and archaea. Encapsulins are virus-like icosahedral particles comprising a ~ 25-50 nm shell surrounding a specific cargo enzyme. Compartmentalization is thought to create a unique chemical environment to facilitate catalysis and isolate toxic intermediates. Many questions regarding nanocompartment structure-function remain unanswered, including how shell symmetry dictates cargo loading and to what extent the shell facilitates enzymatic activity. Here, we explore these questions using the model Thermotoga maritima nanocompartment known to encapsulate a redox-active ferritin-like protein. Biochemical analysis revealed the encapsulin shell to possess a flavin binding site located at the interface between capsomere subunits, suggesting the shell may play a direct and active role in the function of the encapsulated cargo. Furthermore, we used cryo-EM to show that cargo proteins use a form of symmetry-matching to facilitate encapsulation and define stoichiometry. In the case of the Thermotoga maritima encapsulin, the decameric cargo protein with fivefold symmetry preferentially binds to the pentameric-axis of the icosahedral shell. Taken together, these observations suggest the shell is not simply a passive barrier-it also plays a significant role in the structure and function of the cargo enzyme. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24001.map.gz emd_24001.map.gz | 46 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24001-v30.xml emd-24001-v30.xml emd-24001.xml emd-24001.xml | 14.2 KB 14.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24001_fsc.xml emd_24001_fsc.xml | 18.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_24001.png emd_24001.png | 261.3 KB | ||
| Masks |  emd_24001_msk_1.map emd_24001_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-24001.cif.gz emd-24001.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24001 http://ftp.pdbj.org/pub/emdb/structures/EMD-24001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24001 | HTTPS FTP |
-Validation report
| Summary document |  emd_24001_validation.pdf.gz emd_24001_validation.pdf.gz | 485.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24001_full_validation.pdf.gz emd_24001_full_validation.pdf.gz | 485.4 KB | Display | |
| Data in XML |  emd_24001_validation.xml.gz emd_24001_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  emd_24001_validation.cif.gz emd_24001_validation.cif.gz | 22.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24001 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24001 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24001 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24001 | HTTPS FTP |
-Related structure data
| Related structure data |  7mu1MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24001.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24001.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final cryo-EM map for the T maritima encapsulin shell | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_24001_msk_1.map emd_24001_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 3.3A cryo-EM map of the T. maritima encapsulin shell
| Entire | Name: 3.3A cryo-EM map of the T. maritima encapsulin shell |
|---|---|
| Components |
|
-Supramolecule #1: 3.3A cryo-EM map of the T. maritima encapsulin shell
| Supramolecule | Name: 3.3A cryo-EM map of the T. maritima encapsulin shell / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Thermotoga maritima (bacteria) Thermotoga maritima (bacteria) |
| Molecular weight | Theoretical: 1.83 MDa |
-Macromolecule #1: Maritimacin
| Macromolecule | Name: Maritimacin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on peptide bonds (peptidases) |
|---|---|
| Source (natural) | Organism:   Thermotoga maritima (bacteria) Thermotoga maritima (bacteria) |
| Molecular weight | Theoretical: 30.369615 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEFLKRSFAP LTEKQWQEID NRAREIFKTQ LYGRKFVDVE GPYGWEYAAH PLGEVEVLSD ENEVVKWGLR KSLPLIELRA TFTLDLWEL DNLERGKPNV DLSSLEETVR KVAEFEDEVI FRGCEKSGVK GLLSFEERKI ECGSTPKDLL EAIVRALSIF S KDGIEGPY ...String: MEFLKRSFAP LTEKQWQEID NRAREIFKTQ LYGRKFVDVE GPYGWEYAAH PLGEVEVLSD ENEVVKWGLR KSLPLIELRA TFTLDLWEL DNLERGKPNV DLSSLEETVR KVAEFEDEVI FRGCEKSGVK GLLSFEERKI ECGSTPKDLL EAIVRALSIF S KDGIEGPY TLVINTDRWI NFLKEEAGHY PLEKRVEECL RGGKIITTPR IEDALVVSER GGDFKLILGQ DLSIGYEDRE KD AVRLFIT ETFTFQVVNP EALILLK UniProtKB: Type 1 encapsulin shell protein |
-Macromolecule #2: FLAVIN MONONUCLEOTIDE
| Macromolecule | Name: FLAVIN MONONUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 1 / Formula: FMN |
|---|---|
| Molecular weight | Theoretical: 456.344 Da |
| Chemical component information |  ChemComp-FMN: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.0 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 20 mM Tris-HCl, pH 7.4, 150 mM NaCl and 5 mM 2-mercaptoethanol |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot force. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Number real images: 1276 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)