+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20840 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | FACT_subnucleosome complex 1 | |||||||||
 Map data Map data | Sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | assembly / disassembly / transient / integrity / TRANSCRIPTION / TRANSCRIPTION-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationFACT complex / regulation of chromatin organization / positive regulation of DNA-templated transcription, elongation / nucleosome disassembly / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / nucleosome binding / Tat-mediated elongation of the HIV-1 transcript ...FACT complex / regulation of chromatin organization / positive regulation of DNA-templated transcription, elongation / nucleosome disassembly / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / nucleosome binding / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / negative regulation of megakaryocyte differentiation / Formation of HIV elongation complex in the absence of HIV Tat / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / RNA Polymerase II Transcription Elongation / CENP-A containing nucleosome / Formation of RNA Pol II elongation complex / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / nucleosomal DNA binding / RNA Polymerase II Pre-transcription Events / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Inhibition of DNA recombination at telomere / telomere organization / Meiotic synapsis / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Assembly of the ORC complex at the origin of replication / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / SIRT1 negatively regulates rRNA expression / Chromatin modifications during the maternal to zygotic transition (MZT) / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / HCMV Late Events / innate immune response in mucosa / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / HDACs deacetylate histones / TP53 Regulates Transcription of DNA Repair Genes / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / transcription elongation by RNA polymerase II / Formation of the beta-catenin:TCF transactivating complex / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / NoRC negatively regulates rRNA expression / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / G2/M DNA damage checkpoint / HDMs demethylate histones / B-WICH complex positively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / heterochromatin formation / PKMTs methylate histone lysines / Metalloprotease DUBs / Meiotic recombination / Pre-NOTCH Transcription and Translation / RMTs methylate histone arginines / Activation of anterior HOX genes in hindbrain development during early embryogenesis / HCMV Early Events / Transcriptional regulation of granulopoiesis / structural constituent of chromatin / UCH proteinases / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / antibacterial humoral response / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / chromatin organization / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / HATs acetylate histones / Processing of DNA double-strand break ends / histone binding / gene expression / Senescence-Associated Secretory Phenotype (SASP) / Oxidative Stress Induced Senescence / Estrogen-dependent gene expression / Regulation of TP53 Activity through Phosphorylation / transcription by RNA polymerase II / DNA replication / chromosome, telomeric region / Ub-specific processing proteases / defense response to Gram-positive bacterium / cadherin binding / protein heterodimerization activity / Amyloid fiber formation / negative regulation of cell population proliferation / DNA repair / nucleolus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
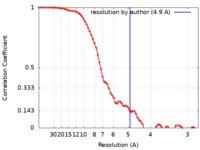
| Method | single particle reconstruction / cryo EM / Resolution: 4.9 Å | |||||||||
 Authors Authors | Zhou K / Tan YZ | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: FACT caught in the act of manipulating the nucleosome. Authors: Yang Liu / Keda Zhou / Naifu Zhang / Hui Wei / Yong Zi Tan / Zhening Zhang / Bridget Carragher / Clinton S Potter / Sheena D'Arcy / Karolin Luger /  Abstract: The organization of genomic DNA into nucleosomes profoundly affects all DNA-related processes in eukaryotes. The histone chaperone known as 'facilitates chromatin transcription' (FACT) (consisting of ...The organization of genomic DNA into nucleosomes profoundly affects all DNA-related processes in eukaryotes. The histone chaperone known as 'facilitates chromatin transcription' (FACT) (consisting of subunits SPT16 and SSRP1) promotes both disassembly and reassembly of nucleosomes during gene transcription, DNA replication and DNA repair. However, the mechanism by which FACT causes these opposing outcomes is unknown. Here we report two cryo-electron-microscopic structures of human FACT in complex with partially assembled subnucleosomes, with supporting biochemical and hydrogen-deuterium exchange data. We find that FACT is engaged in extensive interactions with nucleosomal DNA and all histone variants. The large DNA-binding surface on FACT appears to be protected by the carboxy-terminal domains of both of its subunits, and this inhibition is released by interaction with H2A-H2B, allowing FACT-H2A-H2B to dock onto a complex containing DNA and histones H3 and H4 (ref. ). SPT16 binds nucleosomal DNA and tethers H2A-H2B through its carboxy-terminal domain by acting as a placeholder for DNA. SSRP1 also contributes to DNA binding, and can assume two conformations, depending on whether a second H2A-H2B dimer is present. Our data suggest a compelling mechanism for how FACT maintains chromatin integrity during polymerase passage, by facilitating removal of the H2A-H2B dimer, stabilizing intermediate subnucleosomal states and promoting nucleosome reassembly. Our findings reconcile discrepancies regarding the many roles of FACT and underscore the dynamic interactions between histone chaperones and nucleosomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20840.map.gz emd_20840.map.gz | 28.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20840-v30.xml emd-20840-v30.xml emd-20840.xml emd-20840.xml | 26.8 KB 26.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20840_fsc.xml emd_20840_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_20840.png emd_20840.png | 150.7 KB | ||
| Masks |  emd_20840_msk_1.map emd_20840_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20840.cif.gz emd-20840.cif.gz | 7.7 KB | ||
| Others |  emd_20840_additional.map.gz emd_20840_additional.map.gz emd_20840_half_map_1.map.gz emd_20840_half_map_1.map.gz emd_20840_half_map_2.map.gz emd_20840_half_map_2.map.gz | 9.6 MB 28.3 MB 28.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20840 http://ftp.pdbj.org/pub/emdb/structures/EMD-20840 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20840 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20840 | HTTPS FTP |
-Validation report
| Summary document |  emd_20840_validation.pdf.gz emd_20840_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20840_full_validation.pdf.gz emd_20840_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_20840_validation.xml.gz emd_20840_validation.xml.gz | 13 KB | Display | |
| Data in CIF |  emd_20840_validation.cif.gz emd_20840_validation.cif.gz | 18 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20840 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20840 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20840 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20840 | HTTPS FTP |
-Related structure data
| Related structure data |  6upkMC  6uplC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10333 (Title: Single Particle Cryo-EM Reconstructions of Human FACT in Complex with Partially Assembled Sub-nucleosomes EMPIAR-10333 (Title: Single Particle Cryo-EM Reconstructions of Human FACT in Complex with Partially Assembled Sub-nucleosomesData size: 2.2 TB Data #1: Unaligned multi-frame micrographs [micrographs - multiframe] Data #2: Aligned and dose-weighted micrographs [micrographs - single frame] Data #3: Final Particle Stacks for Class 1 and 2 with Euler Angles and Shifts [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20840.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20840.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.40301 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
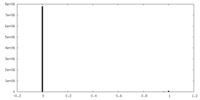
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20840_msk_1.map emd_20840_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
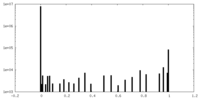
| Density Histograms |
-Additional map: unfiltered map
| File | emd_20840_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unfiltered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
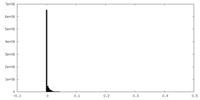
| Density Histograms |
-Half map: #1
| File | emd_20840_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
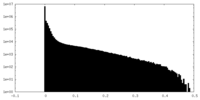
| Density Histograms |
-Half map: #2
| File | emd_20840_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : FACT_subNucleosome_complex_class1
| Entire | Name: FACT_subNucleosome_complex_class1 |
|---|---|
| Components |
|
-Supramolecule #1: FACT_subNucleosome_complex_class1
| Supramolecule | Name: FACT_subNucleosome_complex_class1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 320 KDa |
-Macromolecule #1: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.437167 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEACEA YLVGLFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A
| Macromolecule | Name: Histone H2A / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.135523 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKQGGK ARAKAKSRSS RAGLQFPVGR VHRLLRKGNY AERVGAGAPV YLAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAIR NDEELNKLLG RVTIAQGGVL PNIQAVLLPK KTESHHKAKG K UniProtKB: Histone H2A type 1-C |
-Macromolecule #4: Histone H2B
| Macromolecule | Name: Histone H2B / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.937213 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPEPAKSAPA PKKGSKKAVT KAQKKDGKKR KRSRKESYSV YVYKVLKQVH PDTGISSKAM GIMNSFVNDI FERIAGEASR LAHYNKRST ITSREIQTAV RLLLPGELAK HAVSEGTKAV TKYTSSK UniProtKB: Histone H2B type 1-C/E/F/G/I |
-Macromolecule #5: FACT complex subunit SPT16
| Macromolecule | Name: FACT complex subunit SPT16 / type: protein_or_peptide / ID: 5 Details: Amino acids 640-651 cannot be identified due to the resolution limitations. To show the connectivity of the electron density, poly-(UNK) was placed. This was also done for the end of this ...Details: Amino acids 640-651 cannot be identified due to the resolution limitations. To show the connectivity of the electron density, poly-(UNK) was placed. This was also done for the end of this chain (926-967). The full sequence for the experiment is: MHHHHHHAVTLDKDAYYRRVKRLYSNWRKGEDEYANVDAIVVSVGVDEEIVYAKSTALQTWLFGYELTDTIMVFCDDKII FMASKKKVEFLKQIANTKGNENANGAPAITLLIREKNESNKSSFDKMIEAIKESKNGKKIGVFSKDKFPGEFMKSWNDCL NKEGFDKIDISAVVAYTIAVKEDGELNLMKKAASITSEVFNKFFKERVMEIVDADEKVRHSKLAESVEKAIEEKKYLAGA DPSTVEMCYPPIIQSGGNYNLKFSVVSDKNHMHFGAITCAMGIRFKSYCSNLVRTLMVDPSQEVQENYNFLLQLQEELLK ELRHGVKICDVYNAVMDVVKKQKPELLNKITKNLGFGMGIEFREGSLVINSKNQYKLKKGMVFSINLGFSDLTNKEGKKP EEKTYALFIGDTVLVDEDGPATVLTSVKKKVKNVGIFLKNEDEEEEEEEKDEAEDLLGRGSRAALLTERTRNEMTAEEKR RAHQKELAAQLNEEAKRRLTEQKGEQQIQKARKSNVSYKNPSLMPKEPHIREMKIYIDKKYETVIMPVFGIATPFHIATI KNISMSVEGDYTYLRINFYCPGSALGRNEGNIFPNPEATFVKEITYRASNIKAPGEQTVPALNLQNAFRIIKEVQKRYKT REAEEKEKEGIVKQDSLVINLNRSNPKLKDLYIRPNIAQKRMQGSLEAHVNGFRFTSVRGDKVDILYNNIKHALFQPCDG EMIIVLHFHLKNAIMFGKKRHTDVQFYTEVGEITTDLGKHQHMHDRDDLYAEQMEREMRHKLKTAFKNFIEKVEALTKEE LEFEVPFRDLGFNGAPYRSTCLLQPTSSALVNATEWPPFVVTLDEVELIHFERVQFHLKNFDMVIVYKDYSKKVTMINAI PVASLDPIKEWLNSCDLKYTEGVQSLNWTKIMKTIVDDPEGFFEQGGWSFLEPEGEGSDAEEGDSESEIEDETFNPSEDD YEEEEEDSDEDYSSEAEESDYSKESLGSEEESGKDWDELEEEARKADRESRYEEEEEQSRSMSRKRKASVHSSGRGSNRG SRHSSAPPKKKRK Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 109.574867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHAVT LDKDAYYRRV KRLYSNWRKG EDEYANVDAI VVSVGVDEEI VYAKSTALQT WLFGYELTDT IMVFCDDKII FMASKKKVE FLKQIANTKG NENANGAPAI TLLIREKNES NKSSFDKMIE AIKESKNGKK IGVFSKDKFP GEFMKSWNDC L NKEGFDKI ...String: MHHHHHHAVT LDKDAYYRRV KRLYSNWRKG EDEYANVDAI VVSVGVDEEI VYAKSTALQT WLFGYELTDT IMVFCDDKII FMASKKKVE FLKQIANTKG NENANGAPAI TLLIREKNES NKSSFDKMIE AIKESKNGKK IGVFSKDKFP GEFMKSWNDC L NKEGFDKI DISAVVAYTI AVKEDGELNL MKKAASITSE VFNKFFKERV MEIVDADEKV RHSKLAESVE KAIEEKKYLA GA DPSTVEM CYPPIIQSGG NYNLKFSVVS DKNHMHFGAI TCAMGIRFKS YCSNLVRTLM VDPSQEVQEN YNFLLQLQEE LLK ELRHGV KICDVYNAVM DVVKKQKPEL LNKITKNLGF GMGIEFREGS LVINSKNQYK LKKGMVFSIN LGFSDLTNKE GKKP EEKTY ALFIGDTVLV DEDGPATVLT SVKKKVKNVG IFLKNEDEEE EEEEKDEAED LLGRGSRAAL LTERTRNEMT AEEKR RAHQ KELAAQLNEE AKRRLTEQKG EQQIQKARKS NVSYKNPSLM PKEPHIREMK IYIDKKYETV IMPVFGIATP FHIATI KNI SMSVEGDYTY LRINFYCPGS ALGRNEGNIF PNPEATFVKE ITYRASNIKA PGEQTVPALN LQNAFRIIKE VQKRYK (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)V KQDSLVINLN RSNPKLKDL YIRPNIAQKR MQGSLEAHVN GFRFTSVRGD KVDILYNNIK HALFQPCDGE MIIVLHFHLK NAIMFGKKRH TDVQFYTEVG EITTDLGKH QHMHDRDDLY AEQMEREMRH KLKTAFKNFI EKVEALTKEE LEFEVPFRDL GFNGAPYRST CLLQPTSSAL V NATEWPPF VVTLDEVELI HFERVQFHLK NFDMVIVYKD YSKKVTMINA IPVASLDPIK EWLNSCDLKY TEGVQSLNWT KI MKTIVDD PEGFFEQGGW SFL(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) |
-Macromolecule #6: FACT complex subunit SSRP1
| Macromolecule | Name: FACT complex subunit SSRP1 / type: protein_or_peptide / ID: 6 Details: The poly-(UNK) was placed to show the electron density between 171-198. The full sequence for the experiment is: ...Details: The poly-(UNK) was placed to show the electron density between 171-198. The full sequence for the experiment is: MAETLEFNDVYQEVKGSMNDGRLRLSRQGIIFKNSKTGKVDNIQAGELTEGIWRRVALGHGLKLLTKNGHVYKYDGFRES EFEKLSDFFKTHYRLELMEKDLCVKGWNWGTVKFGGQLLSFDIGDQPVFEIPLSNVSQCTTGKNEVTLEFHQNDDAEVSL MEVRFYVPPTQEDGVDPVEAFAQNVLSKADVIQATGDAICIFRELQCLTPRGRYDIRIYPTFLHLHGKTFDYKIPYTTVL RLFLLPHKDQRQMFFVISLDPPIKQGQTRYHFLILLFSKDEDISLTLNMNEEEVEKRFEGRLTKNMSGSLYEMVSRVMKA LVNRKITVPGNFQGHSGAQCITCSYKASSGLLYPLERGFIYVHKPPVHIRFDEISFVNFARGTTTTRSFDFEIETKQGTQ YTFSSIEREEYGKLFDFVNAKKLNIKNRGLKEGMNPSYDEYADSDEDQHDAYLERMKEEGKIREENANDSSDDSGEETDE SFNPGEEEEDVAEEFDSNASASSSSNEGDSDRDEKKRKQLKKAKMAKDRKSRKKPVEVKKGKDPNAPKRPMSAYMLWLNA SREKIKSDHPGISITDLSKKAGEIWKGMSKEKKEEWDRKAEDARRDYEKAMKEYEGGRGESSKRDKSKKKKKVKVKMEKK STPSRGSSSKSSSRQLSESFKSKEFVSSDESSSGENKSKKKRRRSEDSEEEELASTPPSSEDSASGSDE Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 73.361172 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAETLEFNDV YQEVKGSMND GRLRLSRQGI IFKNSKTGKV DNIQAGELTE GIWRRVALGH GLKLLTKNGH VYKYDGFRES EFEKLSDFF KTHYRLELME KDLCVKGWNW GTVKFGGQLL SFDIGDQPVF EIPLSNVSQC TTGKNEVTLE FHQNDDAEVS L MEVRFYVP ...String: MAETLEFNDV YQEVKGSMND GRLRLSRQGI IFKNSKTGKV DNIQAGELTE GIWRRVALGH GLKLLTKNGH VYKYDGFRES EFEKLSDFF KTHYRLELME KDLCVKGWNW GTVKFGGQLL SFDIGDQPVF EIPLSNVSQC TTGKNEVTLE FHQNDDAEVS L MEVRFYVP PTQ(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)D AICIFRELQC LTPRGRYDI RIYPTFLHLH GKTFDYKIPY TTVLRLFLLP HKDQRQMFFV ISLDPPIKQG QTRYHFLILL FSKDEDISLT LNMNEEEVEK RFEGRLTKN MSGSLYEMVS RVMKALVNRK ITVPGNFQGH SGAQCITCSY KASSGLLYPL ERGFIYVHKP PVHIRFDEIS F VNFARGTT TTRSFDFEIE TKQGTQYTFS SIEREEYGKL FDFVNAKKLN IKNRGLKEGM NPSYDEYADS DEDQHDAYLE RM KEEGKIR EENANDSSDD SGEETDESFN PGEEEEDVAE EFDSNASASS SSNEGDSDRD EKKRKQLKKA KMAKDRKSRK KPV EVKKGK DPNAPKRPMS AYMLWLNASR EKIKSDHPGI SITDLSKKAG EIWKGMSKEK KEEWDRKAED ARRDYEKAMK EYEG GRGES SKRDKSKKKK KVKVKMEKK |
-Macromolecule #7: DNA (79-mer)
| Macromolecule | Name: DNA (79-mer) / type: dna / ID: 7 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 24.144422 KDa |
| Sequence | String: (DT)(DC)(DG)(DT)(DA)(DG)(DA)(DC)(DA)(DG) (DC)(DT)(DC)(DT)(DA)(DG)(DC)(DA)(DC)(DC) (DG)(DC)(DT)(DT)(DA)(DA)(DA)(DC)(DG) (DC)(DA)(DC)(DG)(DT)(DA)(DC)(DG)(DC)(DG) (DC) (DT)(DG)(DT)(DC)(DC)(DC) ...String: (DT)(DC)(DG)(DT)(DA)(DG)(DA)(DC)(DA)(DG) (DC)(DT)(DC)(DT)(DA)(DG)(DC)(DA)(DC)(DC) (DG)(DC)(DT)(DT)(DA)(DA)(DA)(DC)(DG) (DC)(DA)(DC)(DG)(DT)(DA)(DC)(DG)(DC)(DG) (DC) (DT)(DG)(DT)(DC)(DC)(DC)(DC)(DC) (DG)(DC)(DG)(DT)(DT)(DT)(DT)(DA)(DA)(DC) (DC)(DG) (DC)(DC)(DA)(DA)(DG)(DG)(DG) (DG)(DA)(DT)(DT)(DA)(DC)(DT)(DC)(DC)(DC) (DT)(DA) |
-Macromolecule #8: DNA (79-mer)
| Macromolecule | Name: DNA (79-mer) / type: dna / ID: 8 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 24.58468 KDa |
| Sequence | String: (DT)(DA)(DG)(DG)(DG)(DA)(DG)(DT)(DA)(DA) (DT)(DC)(DC)(DC)(DC)(DT)(DT)(DG)(DG)(DC) (DG)(DG)(DT)(DT)(DA)(DA)(DA)(DA)(DC) (DG)(DC)(DG)(DG)(DG)(DG)(DG)(DA)(DC)(DA) (DG) (DC)(DG)(DC)(DG)(DT)(DA) ...String: (DT)(DA)(DG)(DG)(DG)(DA)(DG)(DT)(DA)(DA) (DT)(DC)(DC)(DC)(DC)(DT)(DT)(DG)(DG)(DC) (DG)(DG)(DT)(DT)(DA)(DA)(DA)(DA)(DC) (DG)(DC)(DG)(DG)(DG)(DG)(DG)(DA)(DC)(DA) (DG) (DC)(DG)(DC)(DG)(DT)(DA)(DC)(DG) (DT)(DG)(DC)(DG)(DT)(DT)(DT)(DA)(DA)(DG) (DC)(DG) (DG)(DT)(DG)(DC)(DT)(DA)(DG) (DA)(DG)(DC)(DT)(DG)(DT)(DC)(DT)(DA)(DC) (DG)(DA) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 7.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)