+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
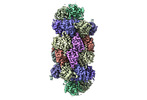
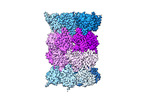
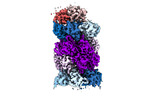
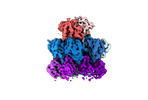
| Title | Human preholo proteasome 20S core particle | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Complex / HYDROLASE | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcerebellar granule cell precursor proliferation / purine ribonucleoside triphosphate binding / protein folding chaperone complex / regulation of endopeptidase activity / proteasome core complex / Regulation of ornithine decarboxylase (ODC) / Cross-presentation of soluble exogenous antigens (endosomes) / Somitogenesis / proteasome core complex assembly / immune system process ...cerebellar granule cell precursor proliferation / purine ribonucleoside triphosphate binding / protein folding chaperone complex / regulation of endopeptidase activity / proteasome core complex / Regulation of ornithine decarboxylase (ODC) / Cross-presentation of soluble exogenous antigens (endosomes) / Somitogenesis / proteasome core complex assembly / immune system process / myofibril / proteasome binding / mitotic spindle assembly checkpoint signaling / NF-kappaB binding / chaperone-mediated protein complex assembly / proteasome assembly / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / proteasome core complex, alpha-subunit complex / threonine-type endopeptidase activity / negative regulation of inflammatory response to antigenic stimulus / : / sarcomere / proteasome complex / Regulation of activated PAK-2p34 by proteasome mediated degradation / ciliary basal body / proteolysis involved in protein catabolic process / Autodegradation of Cdh1 by Cdh1:APC/C / APC/C:Cdc20 mediated degradation of Securin / Asymmetric localization of PCP proteins / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / Ubiquitin-dependent degradation of Cyclin D / AUF1 (hnRNP D0) binds and destabilizes mRNA / TNFR2 non-canonical NF-kB pathway / Assembly of the pre-replicative complex / Vpu mediated degradation of CD4 / Degradation of DVL / Ubiquitin Mediated Degradation of Phosphorylated Cdc25A / Dectin-1 mediated noncanonical NF-kB signaling / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / Hh mutants are degraded by ERAD / lipopolysaccharide binding / Degradation of AXIN / Degradation of GLI1 by the proteasome / Activation of NF-kappaB in B cells / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / Negative regulation of NOTCH4 signaling / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / G2/M Checkpoints / Vif-mediated degradation of APOBEC3G / Autodegradation of the E3 ubiquitin ligase COP1 / Hedgehog 'on' state / Regulation of RUNX3 expression and activity / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / P-body / MAPK6/MAPK4 signaling / APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 / response to virus / Degradation of beta-catenin by the destruction complex / ABC-family proteins mediated transport / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / response to organic cyclic compound / CDK-mediated phosphorylation and removal of Cdc6 / CLEC7A (Dectin-1) signaling / SCF(Skp2)-mediated degradation of p27/p21 / Regulation of expression of SLITs and ROBOs / nuclear matrix / FCERI mediated NF-kB activation / Regulation of PTEN stability and activity / Interleukin-1 signaling / Orc1 removal from chromatin / Regulation of RAS by GAPs / Separation of Sister Chromatids / Regulation of RUNX2 expression and activity / The role of GTSE1 in G2/M progression after G2 checkpoint / UCH proteinases / KEAP1-NFE2L2 pathway / Antigen processing: Ubiquitination & Proteasome degradation / Downstream TCR signaling / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Neddylation / peptidase activity / positive regulation of NF-kappaB transcription factor activity / ER-Phagosome pathway / regulation of inflammatory response / postsynapse / proteasome-mediated ubiquitin-dependent protein catabolic process / secretory granule lumen / endopeptidase activity / response to oxidative stress / ficolin-1-rich granule lumen / molecular adaptor activity / nuclear body / ribosome / Ub-specific processing proteases / nuclear speck Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
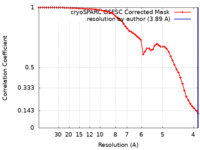
| Method | single particle reconstruction / cryo EM / Resolution: 3.89 Å | |||||||||||||||
 Authors Authors | Schulman BA / Hanna JW / Harper JW / Adolf F / Du J / Rawson SD / Walsh Jr RM / Goodall EA | |||||||||||||||
| Funding support |  Germany, Germany,  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation | Journal: bioRxiv / Year: 2024 Title: Visualizing chaperone-mediated multistep assembly of the human 20S proteasome. Authors: Frank Adolf / Jiale Du / Ellen A Goodall / Richard M Walsh / Shaun Rawson / Susanne von Gronau / J Wade Harper / John Hanna / Brenda A Schulman /   Abstract: Dedicated assembly factors orchestrate stepwise production of many molecular machines, including the 28-subunit proteasome core particle (CP) that mediates protein degradation. Here, we report cryo- ...Dedicated assembly factors orchestrate stepwise production of many molecular machines, including the 28-subunit proteasome core particle (CP) that mediates protein degradation. Here, we report cryo-EM reconstructions of seven recombinant human subcomplexes that visualize all five chaperones and the three active site propeptides across a wide swath of the assembly pathway. Comparison of these chaperone-bound intermediates and a matching mature CP reveals molecular mechanisms determining the order of successive subunit additions, and how proteasome subcomplexes and assembly factors structurally adapt upon progressive subunit incorporation to stabilize intermediates, facilitate the formation of subsequent intermediates, and ultimately rearrange to coordinate proteolytic activation with gated access to active sites. The structural findings reported here explain many previous biochemical and genetic observations. This work establishes a methodologic approach for structural analysis of multiprotein complex assembly intermediates, illuminates specific functions of assembly factors, and reveals conceptual principles underlying human proteasome biogenesis. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18761.map.gz emd_18761.map.gz | 9.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18761-v30.xml emd-18761-v30.xml emd-18761.xml emd-18761.xml | 37.5 KB 37.5 KB | Display Display |  EMDB header EMDB header |
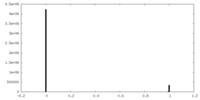
| FSC (resolution estimation) |  emd_18761_fsc.xml emd_18761_fsc.xml | 5.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_18761.png emd_18761.png | 67.2 KB | ||
| Masks |  emd_18761_msk_1.map emd_18761_msk_1.map | 18.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-18761.cif.gz emd-18761.cif.gz | 8.9 KB | ||
| Others |  emd_18761_additional_1.map.gz emd_18761_additional_1.map.gz emd_18761_half_map_1.map.gz emd_18761_half_map_1.map.gz emd_18761_half_map_2.map.gz emd_18761_half_map_2.map.gz | 16.8 MB 16.7 MB 16.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18761 http://ftp.pdbj.org/pub/emdb/structures/EMD-18761 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18761 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18761 | HTTPS FTP |
-Validation report
| Summary document |  emd_18761_validation.pdf.gz emd_18761_validation.pdf.gz | 989.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_18761_full_validation.pdf.gz emd_18761_full_validation.pdf.gz | 988.7 KB | Display | |
| Data in XML |  emd_18761_validation.xml.gz emd_18761_validation.xml.gz | 12.6 KB | Display | |
| Data in CIF |  emd_18761_validation.cif.gz emd_18761_validation.cif.gz | 15.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18761 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18761 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18761 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18761 | HTTPS FTP |
-Related structure data
| Related structure data |  8qysMC  8qyjC  8qylC  8qymC  8qynC  8qyoC  8qz9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18761.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18761.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.885 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_18761_msk_1.map emd_18761_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
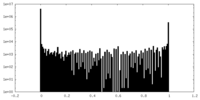
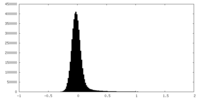
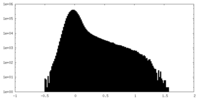
| Density Histograms |
-Additional map: #1
| File | emd_18761_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_18761_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18761_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Human preholo proteasome 20S core particle
+Supramolecule #1: Human preholo proteasome 20S core particle
+Macromolecule #1: Proteasome subunit alpha type-2
+Macromolecule #2: Proteasome subunit alpha type-4
+Macromolecule #3: Proteasome subunit alpha type-7
+Macromolecule #4: Proteasome subunit alpha type-5
+Macromolecule #5: Proteasome subunit alpha type-1
+Macromolecule #6: Proteasome subunit alpha type-3
+Macromolecule #7: Proteasome subunit alpha type-6
+Macromolecule #8: Proteasome maturation protein
+Macromolecule #9: Proteasome assembly chaperone 1
+Macromolecule #10: Proteasome assembly chaperone 2
+Macromolecule #11: Proteasome subunit beta type-7
+Macromolecule #12: Proteasome subunit beta type-3
+Macromolecule #13: Proteasome subunit beta type-2
+Macromolecule #14: Proteasome subunit beta type-5
+Macromolecule #15: Proteasome subunit beta type-1
+Macromolecule #16: Proteasome subunit beta type-4
+Macromolecule #17: Proteasome subunit beta type-6
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.0 µm |
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X

























 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)