+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of Pre-B+5'ss Complex (core part) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | spliceosome / SPLICING | |||||||||
| Function / homology |  Function and homology information Function and homology informationribonucleoprotein complex localization / U4atac snRNP / positive regulation of cytotoxic T cell differentiation / maturation of 5S rRNA / RNA localization / R-loop processing / U4atac snRNA binding / cis assembly of pre-catalytic spliceosome / spliceosome conformational change to release U4 (or U4atac) and U1 (or U11) / snRNP binding ...ribonucleoprotein complex localization / U4atac snRNP / positive regulation of cytotoxic T cell differentiation / maturation of 5S rRNA / RNA localization / R-loop processing / U4atac snRNA binding / cis assembly of pre-catalytic spliceosome / spliceosome conformational change to release U4 (or U4atac) and U1 (or U11) / snRNP binding / U2-type catalytic step 1 spliceosome / RNA splicing, via transesterification reactions / U4 snRNA binding / spliceosomal tri-snRNP complex / U2-type spliceosomal complex / U2-type precatalytic spliceosome / mRNA cis splicing, via spliceosome / U2-type prespliceosome assembly / U2-type catalytic step 2 spliceosome / U4 snRNP / U2 snRNP / U2-type prespliceosome / precatalytic spliceosome / K63-linked polyubiquitin modification-dependent protein binding / mRNA Splicing - Minor Pathway / spliceosomal complex assembly / negative regulation of mRNA splicing, via spliceosome / mRNA 3'-splice site recognition / MLL1 complex / spliceosomal tri-snRNP complex assembly / protein deubiquitination / U5 snRNA binding / U5 snRNP / U2 snRNA binding / U6 snRNA binding / ribonucleoprotein complex binding / spliceosomal snRNP assembly / Cajal body / pre-mRNA intronic binding / U1 snRNA binding / U4/U6 x U5 tri-snRNP complex / catalytic step 2 spliceosome / mRNA Splicing - Major Pathway / RNA splicing / response to cocaine / helicase activity / spliceosomal complex / mRNA splicing, via spliceosome / mRNA processing / osteoblast differentiation / cellular response to xenobiotic stimulus / cellular response to tumor necrosis factor / protein-macromolecule adaptor activity / cellular response to lipopolysaccharide / ubiquitinyl hydrolase 1 / nucleic acid binding / RNA helicase activity / RNA helicase / hydrolase activity / nuclear speck / cell division / intracellular membrane-bounded organelle / GTPase activity / mRNA binding / chromatin / GTP binding / nucleolus / Golgi apparatus / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / RNA binding / extracellular exosome / zinc ion binding / nucleoplasm / ATP binding / identical protein binding / membrane / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
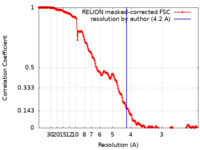
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Zhang Z / Kumar V / Dybkov O / Will CL / Zhong J / Ludwig S / Urlaub H / Kastner B / Stark H / Luehrmann R | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structural insights into the cross-exon to cross-intron spliceosome switch. Authors: Zhenwei Zhang / Vinay Kumar / Olexandr Dybkov / Cindy L Will / Jiayun Zhong / Sebastian E J Ludwig / Henning Urlaub / Berthold Kastner / Holger Stark / Reinhard Lührmann /   Abstract: Early spliceosome assembly can occur through an intron-defined pathway, whereby U1 and U2 small nuclear ribonucleoprotein particles (snRNPs) assemble across the intron. Alternatively, it can occur ...Early spliceosome assembly can occur through an intron-defined pathway, whereby U1 and U2 small nuclear ribonucleoprotein particles (snRNPs) assemble across the intron. Alternatively, it can occur through an exon-defined pathway, whereby U2 binds the branch site located upstream of the defined exon and U1 snRNP interacts with the 5' splice site located directly downstream of it. The U4/U6.U5 tri-snRNP subsequently binds to produce a cross-intron (CI) or cross-exon (CE) pre-B complex, which is then converted to the spliceosomal B complex. Exon definition promotes the splicing of upstream introns and plays a key part in alternative splicing regulation. However, the three-dimensional structure of exon-defined spliceosomal complexes and the molecular mechanism of the conversion from a CE-organized to a CI-organized spliceosome, a pre-requisite for splicing catalysis, remain poorly understood. Here cryo-electron microscopy analyses of human CE pre-B complex and B-like complexes reveal extensive structural similarities with their CI counterparts. The results indicate that the CE and CI spliceosome assembly pathways converge already at the pre-B stage. Add-back experiments using purified CE pre-B complexes, coupled with cryo-electron microscopy, elucidate the order of the extensive remodelling events that accompany the formation of B complexes and B-like complexes. The molecular triggers and roles of B-specific proteins in these rearrangements are also identified. We show that CE pre-B complexes can productively bind in trans to a U1 snRNP-bound 5' splice site. Together, our studies provide new mechanistic insights into the CE to CI switch during spliceosome assembly and its effect on pre-mRNA splice site pairing at this stage. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18555.map.gz emd_18555.map.gz | 440.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18555-v30.xml emd-18555-v30.xml emd-18555.xml emd-18555.xml | 36.6 KB 36.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18555_fsc.xml emd_18555_fsc.xml | 17.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_18555.png emd_18555.png | 44.1 KB | ||
| Filedesc metadata |  emd-18555.cif.gz emd-18555.cif.gz | 13.2 KB | ||
| Others |  emd_18555_half_map_1.map.gz emd_18555_half_map_1.map.gz emd_18555_half_map_2.map.gz emd_18555_half_map_2.map.gz | 380.5 MB 380.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18555 http://ftp.pdbj.org/pub/emdb/structures/EMD-18555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18555 | HTTPS FTP |
-Validation report
| Summary document |  emd_18555_validation.pdf.gz emd_18555_validation.pdf.gz | 873 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_18555_full_validation.pdf.gz emd_18555_full_validation.pdf.gz | 872.6 KB | Display | |
| Data in XML |  emd_18555_validation.xml.gz emd_18555_validation.xml.gz | 24.6 KB | Display | |
| Data in CIF |  emd_18555_validation.cif.gz emd_18555_validation.cif.gz | 32.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18555 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18555 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18555 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18555 | HTTPS FTP |
-Related structure data
| Related structure data |  8qpkMC  8qozC  8qp8C  8qp9C  8qpaC  8qpbC  8qpeC  8qxdC  8qzsC  8r08C  8r09C  8r0aC  8r0bC  8rm5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18555.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18555.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_18555_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18555_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : human spliceosomal pre-B+5'ss complex
+Supramolecule #1: human spliceosomal pre-B+5'ss complex
+Macromolecule #1: Probable ATP-dependent RNA helicase DDX23
+Macromolecule #2: Thioredoxin-like protein 4A
+Macromolecule #3: U4/U6 small nuclear ribonucleoprotein Prp31
+Macromolecule #4: Pre-mRNA-processing-splicing factor 8
+Macromolecule #5: RNA-binding protein 42
+Macromolecule #6: Ubiquitin carboxyl-terminal hydrolase 39
+Macromolecule #7: U4/U6.U5 tri-snRNP-associated protein 1
+Macromolecule #8: 116 kDa U5 small nuclear ribonucleoprotein component
+Macromolecule #11: U4/U6.U5 small nuclear ribonucleoprotein 27 kDa protein
+Macromolecule #14: Pre-mRNA-processing factor 6
+Macromolecule #15: U5 small nuclear ribonucleoprotein 200 kDa helicase
+Macromolecule #16: Splicing factor 3A subunit 1
+Macromolecule #9: U5 snRNA
+Macromolecule #10: U4 snRNA
+Macromolecule #12: 5'ss oligo
+Macromolecule #13: U6 snRNA
+Macromolecule #17: INOSITOL HEXAKISPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)