+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the type I-G CRISPR effector | |||||||||
 Map data Map data | Map from the Csx17 Multibody segment | |||||||||
 Sample Sample |
| |||||||||
| Function / homology | CRISPR-associated protein Csx17, subtype Dpsyc / Type I-U CRISPR-associated protein Csx17 Function and homology information Function and homology information | |||||||||
| Biological species |  Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria) Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 8.0 Å cryo EM / Resolution: 8.0 Å | |||||||||
 Authors Authors | Shangguan Q / Graham S / Sundaramoorthy R / White MF | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: Structure and mechanism of the type I-G CRISPR effector. Authors: Qilin Shangguan / Shirley Graham / Ramasubramanian Sundaramoorthy / Malcolm F White /  Abstract: Type I CRISPR systems are the most common CRISPR type found in bacteria. They use a multisubunit effector, guided by crRNA, to detect and bind dsDNA targets, forming an R-loop and recruiting the Cas3 ...Type I CRISPR systems are the most common CRISPR type found in bacteria. They use a multisubunit effector, guided by crRNA, to detect and bind dsDNA targets, forming an R-loop and recruiting the Cas3 enzyme to facilitate target DNA destruction, thus providing immunity against mobile genetic elements. Subtypes have been classified into families A-G, with type I-G being the least well understood. Here, we report the composition, structure and function of the type I-G Cascade CRISPR effector from Thioalkalivibrio sulfidiphilus, revealing key new molecular details. The unique Csb2 subunit processes pre-crRNA, remaining bound to the 3' end of the mature crRNA, and seven Cas7 subunits form the backbone of the effector. Cas3 associates stably with the effector complex via the Cas8g subunit and is important for target DNA recognition. Structural analysis by cryo-Electron Microscopy reveals a strikingly curved backbone conformation with Cas8g spanning the belly of the structure. These biochemical and structural insights shed new light on the diversity of type I systems and open the way to applications in genome engineering. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15820.map.gz emd_15820.map.gz | 6.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15820-v30.xml emd-15820-v30.xml emd-15820.xml emd-15820.xml | 20 KB 20 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_15820.png emd_15820.png | 101.1 KB | ||
| Others |  emd_15820_half_map_1.map.gz emd_15820_half_map_1.map.gz emd_15820_half_map_2.map.gz emd_15820_half_map_2.map.gz | 120.4 MB 120.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15820 http://ftp.pdbj.org/pub/emdb/structures/EMD-15820 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15820 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15820 | HTTPS FTP |
-Related structure data
| Related structure data |  8b2xMC  8aneC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15820.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15820.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map from the Csx17 Multibody segment | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.008 Å | ||||||||||||||||||||||||||||||||||||
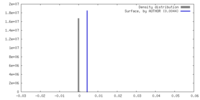
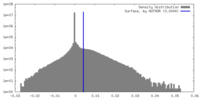
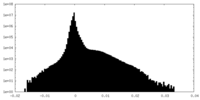
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map from the Csx17 Multibody segment
| File | emd_15820_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map from the Csx17 Multibody segment | ||||||||||||
| Projections & Slices |
| ||||||||||||
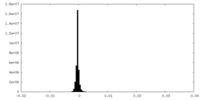
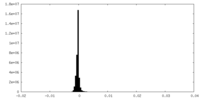
| Density Histograms |
-Half map: Half Map from the Csx17 Multibody segment
| File | emd_15820_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map from the Csx17 Multibody segment | ||||||||||||
| Projections & Slices |
| ||||||||||||
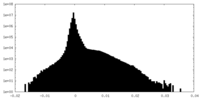
| Density Histograms |
- Sample components
Sample components
-Entire : Type I-G cascade complex large subunit CSX17 protein
| Entire | Name: Type I-G cascade complex large subunit CSX17 protein |
|---|---|
| Components |
|
-Supramolecule #1: Type I-G cascade complex large subunit CSX17 protein
| Supramolecule | Name: Type I-G cascade complex large subunit CSX17 protein / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria) Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 7.926 KDa |
-Macromolecule #1: Type I-G CRISPR Cascade large subunit CSX17
| Macromolecule | Name: Type I-G CRISPR Cascade large subunit CSX17 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria) Thioalkalivibrio sulfidiphilus HL-EbGr7 (bacteria)Strain: HL-EbGR7 |
| Molecular weight | Theoretical: 79.372023 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MDKDMHINEI VLRGCAPTPL AAYLKALGVL RLVCEQVDAT AKGWWQDECF MLRTRLDDND LRRFFIEDYR PTPMLSPWNG GSGFYRKGN ETAWSTLEKI ITTQAERWRP FRDTAEVMAD ALEHLKLTEK PAELDKRALL ARLRATLDDE FLPWLDAAVL L TDDKPDYP ...String: MDKDMHINEI VLRGCAPTPL AAYLKALGVL RLVCEQVDAT AKGWWQDECF MLRTRLDDND LRRFFIEDYR PTPMLSPWNG GSGFYRKGN ETAWSTLEKI ITTQAERWRP FRDTAEVMAD ALEHLKLTEK PAELDKRALL ARLRATLDDE FLPWLDAAVL L TDDKPDYP PLLGTGGNDG RLDFTSNYMQ RLLEMFDPVT GKAQGDVGNK LESALFARPV PGMTALAIGQ FSPGAAGGPN SS TGFDSGA QVNIWDYVLM LEGALLFAAT ATRRLESADP SALSYPFTVR PSGGGSGAVA LGDERPARAE IWMPLWERPA SLP ELRVLL GEGRVTLNGR LPRDGLDFAR AVAKLGTDRG VRAFQRYAFM MRSGKAYLAT PLNRFHVHRN PKADLIDQLE RGDW LRRFR RAARSTHAPA RLQGLAHRLD DALFDLVRVA DPRRVQEVLK VLGEVQFYLA LSPSLREQVR PVPRLDAHWV EAARD DSHE FRVAAALAGL DDGLPMGVHL APIDPVKRNV WAPESRLAVW GQGNLSDNLA QVLQRRLLTA SRTDLNDKPL SGRCPA DEG AVAAFLAGDA DERRIAELMA GLACARLPAR LPLRQRGASE ASSLPMIYAL LKPLFVPDAQ LREAAVLTPD GCLPLPP AL PRLLRAGPAG VGRAVDLARR RRRASGLADA GWRLTPPYPD GGRLLAALMI PVEIRVIKGF IKRLADHKSD EPATQDAS |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20mM Tris-HCl, 250mM NaCl, pH 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.0001 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 4 K / Instrument: FEI VITROBOT MARK IV / Details: blot time 3.5, blot force 4. |
| Details | Size exclusion purified monodisperse sample in solution |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 2.8000000000000003 µm / Calibrated defocus min: 1.6 µm / Calibrated magnification: 105000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.6 µm / Nominal magnification: 105000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.6 µm / Nominal magnification: 105000 |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Temperature | Min: 77.0 K / Max: 183.0 K |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5000 pixel / Digitization - Dimensions - Height: 4000 pixel / Digitization - Sampling interval: 0.84 µm / Number grids imaged: 1 / Number real images: 15710 / Average exposure time: 3.0 sec. / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 1000 |
|---|---|
| CTF correction | Software - Name: CTFFIND (ver. 4) |
| Startup model | Type of model: INSILICO MODEL / In silico model: RELION INITIAL MODEL / Details: Using stochastic gradient method |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: RELION (ver. 4) |
| Final 3D classification | Number classes: 2 / Avg.num./class: 2300000 / Software - Name: RELION (ver. 4) |
| Final angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: RELION (ver. 4) |
| Final reconstruction | Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 8.0 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 4) / Details: Multi-body refinement. / Number images used: 415000 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 188 / Target criteria: Correlation Coefficient |
|---|---|
| Output model |  PDB-8b2x: |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)