+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human BIRC6 in complex with HTRA2 | |||||||||
 Map data Map data | B factor blurred 200 A2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E3 ubiquitin ligase / E2/E3 hybrid / inhibitor of apoptosis protein / LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationHtrA2 peptidase / pentacyclic triterpenoid metabolic process / : / spongiotrophoblast layer development / ceramide metabolic process / regulation of autophagy of mitochondrion / mitochondrial protein catabolic process / CD40 receptor complex / labyrinthine layer development / ALK mutants bind TKIs ...HtrA2 peptidase / pentacyclic triterpenoid metabolic process / : / spongiotrophoblast layer development / ceramide metabolic process / regulation of autophagy of mitochondrion / mitochondrial protein catabolic process / CD40 receptor complex / labyrinthine layer development / ALK mutants bind TKIs / programmed cell death / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / Flemming body / protein serine/threonine kinase inhibitor activity / serine-type endopeptidase complex / microtubule organizing center / adult walking behavior / positive regulation of protein targeting to mitochondrion / response to herbicide / cysteine-type endopeptidase inhibitor activity / execution phase of apoptosis / : / positive regulation of execution phase of apoptosis / ubiquitin conjugating enzyme activity / protein autoprocessing / ubiquitin ligase inhibitor activity / negative regulation of cell cycle / regulation of multicellular organism growth / neuron development / cellular response to interferon-beta / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / cellular response to retinoic acid / forebrain development / Mitochondrial protein degradation / serine-type peptidase activity / regulation of cytokinesis / mitochondrion organization / negative regulation of extrinsic apoptotic signaling pathway / mitochondrial membrane / trans-Golgi network / RING-type E3 ubiquitin transferase / protein catabolic process / cellular response to growth factor stimulus / mitochondrial intermembrane space / spindle pole / cytoplasmic side of plasma membrane / intrinsic apoptotic signaling pathway in response to DNA damage / ubiquitin-protein transferase activity / unfolded protein binding / Signaling by ALK fusions and activated point mutants / peptidase activity / regulation of cell population proliferation / cellular response to heat / cellular response to oxidative stress / midbody / neuron apoptotic process / negative regulation of neuron apoptotic process / cell population proliferation / cytoskeleton / intracellular signal transduction / endosome / protein ubiquitination / positive regulation of apoptotic process / protein phosphorylation / cell division / serine-type endopeptidase activity / centrosome / positive regulation of cell population proliferation / endoplasmic reticulum membrane / chromatin / negative regulation of apoptotic process / apoptotic process / endoplasmic reticulum / mitochondrion / proteolysis / identical protein binding / membrane / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
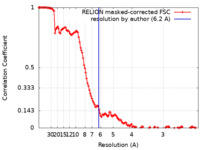
| Method | single particle reconstruction / cryo EM / Resolution: 6.2 Å | |||||||||
 Authors Authors | Ehrmann JF / Grabarczyk DB / Clausen T | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Structural basis for regulation of apoptosis and autophagy by the BIRC6/SMAC complex. Authors: Julian F Ehrmann / Daniel B Grabarczyk / Maria Heinke / Luiza Deszcz / Robert Kurzbauer / Otto Hudecz / Alexandra Shulkina / Rebeca Gogova / Anton Meinhart / Gijs A Versteeg / Tim Clausen /  Abstract: Inhibitor of apoptosis proteins (IAPs) bind to pro-apoptotic proteases, keeping them inactive and preventing cell death. The atypical ubiquitin ligase BIRC6 is the only essential IAP, additionally ...Inhibitor of apoptosis proteins (IAPs) bind to pro-apoptotic proteases, keeping them inactive and preventing cell death. The atypical ubiquitin ligase BIRC6 is the only essential IAP, additionally functioning as a suppressor of autophagy. We performed a structure-function analysis of BIRC6 in complex with caspase-9, HTRA2, SMAC, and LC3B, which are critical apoptosis and autophagy proteins. Cryo-electron microscopy structures showed that BIRC6 forms a megadalton crescent shape that arcs around a spacious cavity containing receptor sites for client proteins. Multivalent binding of SMAC obstructs client binding, impeding ubiquitination of both autophagy and apoptotic substrates. On the basis of these data, we discuss how the BIRC6/SMAC complex can act as a stress-induced hub to regulate apoptosis and autophagy drivers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15672.map.gz emd_15672.map.gz | 168.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15672-v30.xml emd-15672-v30.xml emd-15672.xml emd-15672.xml | 25.6 KB 25.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15672_fsc.xml emd_15672_fsc.xml | 13 KB | Display |  FSC data file FSC data file |
| Images |  emd_15672.png emd_15672.png | 65 KB | ||
| Masks |  emd_15672_msk_1.map emd_15672_msk_1.map | 184 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15672.cif.gz emd-15672.cif.gz | 9.2 KB | ||
| Others |  emd_15672_additional_1.map.gz emd_15672_additional_1.map.gz emd_15672_additional_2.map.gz emd_15672_additional_2.map.gz emd_15672_half_map_1.map.gz emd_15672_half_map_1.map.gz emd_15672_half_map_2.map.gz emd_15672_half_map_2.map.gz | 171.1 MB 170.5 MB 146.6 MB 146.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15672 http://ftp.pdbj.org/pub/emdb/structures/EMD-15672 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15672 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15672 | HTTPS FTP |
-Validation report
| Summary document |  emd_15672_validation.pdf.gz emd_15672_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15672_full_validation.pdf.gz emd_15672_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_15672_validation.xml.gz emd_15672_validation.xml.gz | 20.2 KB | Display | |
| Data in CIF |  emd_15672_validation.cif.gz emd_15672_validation.cif.gz | 26.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15672 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15672 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15672 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15672 | HTTPS FTP |
-Related structure data
| Related structure data |  8aukMC  8atuC  8atxC  8auwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15672.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15672.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B factor blurred 200 A2 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.17 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15672_msk_1.map emd_15672_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map low-pass filtered to 8 A
| File | emd_15672_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map low-pass filtered to 8 A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map low-pass filtered to 10 A
| File | emd_15672_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map low-pass filtered to 10 A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_15672_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15672_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human BIRC6 homodimer in complex with HTRA2 homotrimer
| Entire | Name: Human BIRC6 homodimer in complex with HTRA2 homotrimer |
|---|---|
| Components |
|
-Supramolecule #1: Human BIRC6 homodimer in complex with HTRA2 homotrimer
| Supramolecule | Name: Human BIRC6 homodimer in complex with HTRA2 homotrimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.17 MDa |
-Macromolecule #1: Baculoviral IAP repeat-containing protein 6
| Macromolecule | Name: Baculoviral IAP repeat-containing protein 6 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: RING-type E3 ubiquitin transferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 532.009 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MVTGGGAAPP GTVTEPLPSV IVLSAGRKMA AAAAAASGPG CSSAAGAGAA GVSEWLVLRD GCMHCDADGL HSLSYHPALN AILAVTSRG TIKVIDGTSG ATLQASALSA KPGGQVKCQY ISAVDKVIFV DDYAVGCRKD LNGILLLDTA LQTPVSKQDD V VQLELPVT ...String: MVTGGGAAPP GTVTEPLPSV IVLSAGRKMA AAAAAASGPG CSSAAGAGAA GVSEWLVLRD GCMHCDADGL HSLSYHPALN AILAVTSRG TIKVIDGTSG ATLQASALSA KPGGQVKCQY ISAVDKVIFV DDYAVGCRKD LNGILLLDTA LQTPVSKQDD V VQLELPVT EAQQLLSACL EKVDISSTEG YDLFITQLKD GLKNTSHETA ANHKVAKWAT VTFHLPHHVL KSIASAIVNE LK KINQNVA ALPVASSVMD RLSYLLPSAR PELGVGPGRS VDRSLMYSEA NRRETFTSWP HVGYRWAQPD PMAQAGFYHQ PAS SGDDRA MCFTCSVCLV CWEPTDEPWS EHERHSPNCP FVKGEHTQNV PLSVTLATSP AQFPCTDGTD RISCFGSGSC PHFL AAATK RGKICIWDVS KLMKVHLKFE INAYDPAIVQ QLILSGDPSS GVDSRRPTLA WLEDSSSCSD IPKLEGDSDD LLEDS DSEE HSRSDSVTGH TSQKEAMEVS LDITALSILQ QPEKLQWEIV ANVLEDTVKD LEELGANPCL TNSKSEKTKE KHQEQH NIP FPCLLAGGLL TYKSPATSPI SSNSHRSLDG LSRTQGESIS EQGSTDNESC TNSELNSPLV RRTLPVLLLY SIKESDE KA GKIFSQMNNI MSKSLHDDGF TVPQIIEMEL DSQEQLLLQD PPVTYIQQFA DAAANLTSPD SEKWNSVFPK PGTLVQCL R LPKFAEEENL CIDSITPCAD GIHLLVGLRT CPVESLSAIN QVEALNNLNK LNSALCNRRK GELESNLAVV NGANISVIQ HESPADVQTP LIIQPEQRNV SGGYLVLYKM NYATRIVTLE EEPIKIQHIK DPQDTITSLI LLPPDILDNR EDDCEEPIED MQLTSKNGF EREKTSDIST LGHLVITTQG GYVKILDLSN FEILAKVEPP KKEGTEEQDT FVSVIYCSGT DRLCACTKGG E LHFLQIGG TCDDIDEADI LVDGSLSKGI EPSSEGSKPL SNPSSPGISG VDLLVDQPFT LEILTSLVEL TRFETLTPRF SA TVPPCWV EVQQEQQQRR HPQHLHQQHH GDAAQHTRTW KLQTDSNSWD EHVFELVLPK ACMVGHVDFK FVLNSNITNI PQI QVTLLK NKAPGLGKVN ALNIEVEQNG KPSLVDLNEE MQHMDVEESQ CLRLCPFLED HKEDILCGPV WLASGLDLSG HAGM LTLTS PKLVKGMAGG KYRSFLIHVK AVNERGTEEI CNGGMRPVVR LPSLKHQSNK GYSLASLLAK VAAGKEKSSN VKNEN TSGT RKSENLRGCD LLQEVSVTIR RFKKTSISKE RVQRCAMLQF SEFHEKLVNT LCRKTDDGQI TEHAQSLVLD TLCWLA GVH SNGPGSSKEG NENLLSKTRK FLSDIVRVCF FEAGRSIAHK CARFLALCIS NGKCDPCQPA FGPVLLKALL DNMSFLP AA TTGGSVYWYF VLLNYVKDED LAGCSTACAS LLTAVSRQLQ DRLTPMEALL QTRYGLYSSP FDPVLFDLEM SGSSCKNV Y NSSIGVQSDE IDLSDVLSGN GKVSSCTAAE GSFTSLTGLL EVEPLHFTCV STSDGTRIER DDAMSSFGVT PAVGGLSSG TVGEASTALS SAAQVALQSL SHAMASAEQQ LQVLQEKQQQ LLKLQQQKAK LEAKLHQTTA AAAAAASAVG PVHNSVPSNP VAAPGFFIH PSDVIPPTPK TTPLFMTPPL TPPNEAVSVV INAELAQLFP GSVIDPPAVN LAAHNKNSNK SRMNPLGSGL A LAISHASH FLQPPPHQSI IIERMHSGAR RFVTLDFGRP ILLTDVLIPT CGDLASLSID IWTLGEEVDG RRLVVATDIS TH SLILHDL IPPPVCRFMK ITVIGRYGST NARAKIPLGF YYGHTYILPW ESELKLMHDP LKGEGESANQ PEIDQHLAMM VAL QEDIQC RYNLACHRLE TLLQSIDLPP LNSANNAQYF LRKPDKAVEE DSRVFSAYQD CIQLQLQLNL AHNAVQRLKV ALGA SRKML SETSNPEDLI QTSSTEQLRT IIRYLLDTLL SLLHASNGHS VPAVLQSTFH AQACEELFKH LCISGTPKIR LHTGL LLVQ LCGGERWWGQ FLSNVLQELY NSEQLLIFPQ DRVFMLLSCI GQRSLSNSGV LESLLNLLDN LLSPLQPQLP MHRRTE GVL DIPMISWVVM LVSRLLDYVA TVEDEAAAAK KPLNGNQWSF INNNLHTQSL NRSSKGSSSL DRLYSRKIRK QLVHHKQ QL NLLKAKQKAL VEQMEKEKIQ SNKGSSYKLL VEQAKLKQAT SKHFKDLIRL RRTAEWSRSN LDTEVTTAKE SPEIEPLP F TLAHERCISV VQKLVLFLLS MDFTCHADLL LFVCKVLARI ANATRPTIHL CEIVNEPQLE RLLLLLVGTD FNRGDISWG GAWAQYSLTC MLQDILAGEL LAPVAAEAME EGTVGDDVGA TAGDSDDSLQ QSSVQLLETI DEPLTHDITG APPLSSLEKD KEIDLELLQ DLMEVDIDPL DIDLEKDPLA AKVFKPISST WYDYWGADYG TYNYNPYIGG LGIPVAKPPA NTEKNGSQTV S VSVSQALD ARLEVGLEQQ AELMLKMMST LEADSILQAL TNTSPTLSQS PTGTDDSLLG GLQAANQTSQ LIIQLSSVPM LN VCFNKLF SMLQVHHVQL ESLLQLWLTL SLNSSSTGNK ENGADIFLYN ANRIPVISLN QASITSFLTV LAWYPNTLLR TWC LVLHSL TLMTNMQLNS GSSSAIGTQE STAHLLVSDP NLIHVLVKFL SGTSPHGTNQ HSPQVGPTAT QAMQEFLTRL QVHL SSTCP QIFSEFLLKL IHILSTERGA FQTGQGPLDA QVKLLEFTLE QNFEVVSVST ISAVIESVTF LVHHYITCSD KVMSR SGSD SSVGARACFG GLFANLIRPG DAKAVCGEMT RDQLMFDLLK LVNILVQLPL SGNREYSARV SVTTNTTDSV SDEEKV SGG KDGNGSSTSV QGSPAYVADL VLANQQIMSQ ILSALGLCNS SAMAMIIGAS GLHLTKHENF HGGLDAISVG DGLFTIL TT LSKKASTVHM MLQPILTYMA CGYMGRQGSL ATCQLSEPLL WFILRVLDTS DALKAFHDMG GVQLICNNMV TSTRAIVN T ARSMVSTIMK FLDSGPNKAV DSTLKTRILA SEPDNAEGIH NFAPLGTITS SSPTAQPAEV LLQATPPHRR ARSAAWSYI FLPEEAWCDL TIHLPAAVLL KEIHIQPHLA SLATCPSSVS VEVSADGVNM LPLSTPVVTS GLTYIKIQLV KAEVASAVCL RLHRPRDAS TLGLSQIKLL GLTAFGTTSS ATVNNPFLPS EDQVSKTSIG WLRLLHHCLT HISDLEGMMA SAAAPTANLL Q TCAALLMS PYCGMHSPNI EVVLVKIGLQ STRIGLKLID ILLRNCAASG SDPTDLNSPL LFGRLNGLSS DSTIDILYQL GT TQDPGTK DRIQALLKWV SDSARVAAMK RSGRMNYMCP NSSTVEYGLL MPSPSHLHCV AAILWHSYEL LVEYDLPALL DQE LFELLF NWSMSLPCNM VLKKAVDSLL CSMCHVHPNY FSLLMGWMGI TPPPVQCHHR LSMTDDSKKQ DLSSSLTDDS KNAQ APLAL TESHLATLAS SSQSPEAIKQ LLDSGLPSLL VRSLASFCFS HISSSESIAQ SIDISQDKLR RHHVPQQCNK MPITA DLVA PILRFLTEVG NSHIMKDWLG GSEVNPLWTA LLFLLCHSGS TSGSHNLGAQ QTSARSASLS SAATTGLTTQ QRTAIE NAT VAFFLQCISC HPNNQKLMAQ VLCELFQTSP QRGNLPTSGN ISGFIRRLFL QLMLEDEKVT MFLQSPCPLY KGRINAT SH VIQHPMYGAG HKFRTLHLPV STTLSDVLDR VSDTPSITAK LISEQKDDKE KKNHEEKEKV KAENGFQDNY SVVVASGL K SQSKRAVSAT PPRPPSRRGR TIPDKIGSTS GAEAANKIIT VPVFHLFHKL LAGQPLPAEM TLAQLLTLLY DRKLPQGYR SIDLTVKLGS RVITDPSLSK TDSYKRLHPE KDHGDLLASC PEDEALTPGD ECMDGILDES LLETCPIQSP LQVFAGMGGL ALIAERLPM LYPEVIQQVS APVVTSTTQE KPKDSDQFEW VTIEQSGELV YEAPETVAAE PPPIKSAVQT MSPIPAHSLA A FGLFLRLP GYAEVLLKER KHAQCLLRLV LGVTDDGEGS HILQSPSANV LPTLPFHVLR SLFSTTPLTT DDGVLLRRMA LE IGALHLI LVCLSALSHH SPRVPNSSVN QTEPQVSSSH NPTSTEEQQL YWAKGTGFGT GSTASGWDVE QALTKQRLEE EHV TCLLQV LASYINPVSS AVNGEAQSSH ETRGQNSNAL PSVLLELLSQ SCLIPAMSSY LRNDSVLDMA RHVPLYRALL ELLR AIASC AAMVPLLLPL STENGEEEEE QSECQTSVGT LLAKMKTCVD TYTNRLRSKR ENVKTGVKPD ASDQEPEGLT LLVPD IQKT AEIVYAATTS LRQANQEKKL GEYSKKAAMK PKPLSVLKSL EEKYVAVMKK LQFDTFEMVS EDEDGKLGFK VNYHYM SQV KNANDANSAA RARRLAQEAV TLSTSLPLSS SSSVFVRCDE ERLDIMKVLI TGPADTPYAN GCFEFDVYFP QDYPSSP PL VNLETTGGHS VRFNPNLYND GKVCLSILNT WHGRPEEKWN PQTSSFLQVL VSVQSLILVA EPYFNEPGYE RSRGTPSG T QSSREYDGNI RQATVKWAML EQIRNPSPCF KEVIHKHFYL KRVEIMAQCE EWIADIQQYS SDKRVGRTMS HHAAALKRH TAQLREELLK LPCPEGLDPD TDDAPEVCRA TTGAEETLMH DQVKPSSSKE LPSDFQLSAW SHPQFEK UniProtKB: Baculoviral IAP repeat-containing protein 6 |
-Macromolecule #2: Serine protease HTRA2, mitochondrial
| Macromolecule | Name: Serine protease HTRA2, mitochondrial / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number: HtrA2 peptidase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 35.00182 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AVPSPPPASP RSQYNFIADV VEKTAPAVVY IEILDRHPFL GREVPISNGS GFVVAADGLI VTNAHVVADR RRVRVRLLSG DTYEAVVTA VDPVADIATL RIQTKEPLPT LPLGRSADVR QGEFVVAMGS PFALQNTITS GIVSSAQRPA RDLGLPQTNV E YIQTDAAI ...String: AVPSPPPASP RSQYNFIADV VEKTAPAVVY IEILDRHPFL GREVPISNGS GFVVAADGLI VTNAHVVADR RRVRVRLLSG DTYEAVVTA VDPVADIATL RIQTKEPLPT LPLGRSADVR QGEFVVAMGS PFALQNTITS GIVSSAQRPA RDLGLPQTNV E YIQTDAAI DFGNAGGPLV NLDGEVIGVN TMKVTAGISF AIPSDRLREF LHRGEKKNSS SGISGSQRRY IGVMMLTLSP SI LAELQLR EPSFPDVQHG VLIHKVILGS PAHRAGLRPG DVILAIGEQM VQNAEDVYEA VRTQSQLAVQ IRRGRETLTL YVT PEVTE UniProtKB: Serine protease HTRA2, mitochondrial |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)