[English] 日本語
 Yorodumi
Yorodumi- EMDB-12707: O-Layer C14 at 2.58A - Local refinement with C14 symmetry of the ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | O-Layer C14 at 2.58A - Local refinement with C14 symmetry of the O-layer of the outer membrane core complex from the fully-assembled R388 type IV secretion system. | |||||||||||||||
 Map data Map data | O-Layer C14 Local Refinement map Sharpened | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.6 Å cryo EM / Resolution: 2.6 Å | |||||||||||||||
 Authors Authors | Mace K / Vadakkepat AK / Lukoyanova N / Waksman G | |||||||||||||||
| Funding support |  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Cryo-EM structure of a type IV secretion system. Authors: Kévin Macé / Abhinav K Vadakkepat / Adam Redzej / Natalya Lukoyanova / Clasien Oomen / Nathalie Braun / Marta Ukleja / Fang Lu / Tiago R D Costa / Elena V Orlova / David Baker / Qian Cong ...Authors: Kévin Macé / Abhinav K Vadakkepat / Adam Redzej / Natalya Lukoyanova / Clasien Oomen / Nathalie Braun / Marta Ukleja / Fang Lu / Tiago R D Costa / Elena V Orlova / David Baker / Qian Cong / Gabriel Waksman /     Abstract: Bacterial conjugation is the fundamental process of unidirectional transfer of DNAs, often plasmid DNAs, from a donor cell to a recipient cell. It is the primary means by which antibiotic resistance ...Bacterial conjugation is the fundamental process of unidirectional transfer of DNAs, often plasmid DNAs, from a donor cell to a recipient cell. It is the primary means by which antibiotic resistance genes spread among bacterial populations. In Gram-negative bacteria, conjugation is mediated by a large transport apparatus-the conjugative type IV secretion system (T4SS)-produced by the donor cell and embedded in both its outer and inner membranes. The T4SS also elaborates a long extracellular filament-the conjugative pilus-that is essential for DNA transfer. Here we present a high-resolution cryo-electron microscopy (cryo-EM) structure of a 2.8 megadalton T4SS complex composed of 92 polypeptides representing 8 of the 10 essential T4SS components involved in pilus biogenesis. We added the two remaining components to the structural model using co-evolution analysis of protein interfaces, to enable the reconstitution of the entire system including the pilus. This structure describes the exceptionally large protein-protein interaction network required to assemble the many components that constitute a T4SS and provides insights on the unique mechanism by which they elaborate pili. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12707.map.gz emd_12707.map.gz | 17.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12707-v30.xml emd-12707-v30.xml emd-12707.xml emd-12707.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12707.png emd_12707.png | 106.6 KB | ||
| Masks |  emd_12707_msk_1.map emd_12707_msk_1.map | 18.9 MB |  Mask map Mask map | |
| Others |  emd_12707_additional_1.map.gz emd_12707_additional_1.map.gz emd_12707_half_map_1.map.gz emd_12707_half_map_1.map.gz emd_12707_half_map_2.map.gz emd_12707_half_map_2.map.gz | 17.1 MB 94.4 MB 94.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12707 http://ftp.pdbj.org/pub/emdb/structures/EMD-12707 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12707 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12707 | HTTPS FTP |
-Related structure data
| Related structure data |  7o3jMC  7o3tC  7o3vC  7o41C  7o42C  7o43C  7oiuC  7q1vC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12707.map.gz / Format: CCP4 / Size: 18.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12707.map.gz / Format: CCP4 / Size: 18.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | O-Layer C14 Local Refinement map Sharpened | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.067 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_12707_msk_1.map emd_12707_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
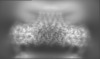
| Projections & Slices |
| ||||||||||||
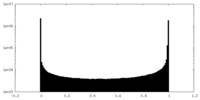
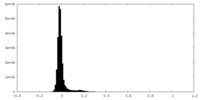
| Density Histograms |
-Additional map: O-Layer C14 Local Refinement map Unsharpened
| File | emd_12707_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | O-Layer C14 Local Refinement map Unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
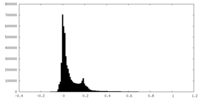
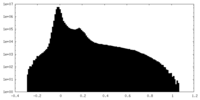
| Density Histograms |
-Half map: O-Layer C14 Local Refinement - Half-A
| File | emd_12707_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | O-Layer C14 Local Refinement - Half-A | ||||||||||||
| Projections & Slices |
| ||||||||||||
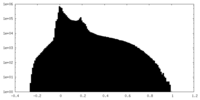
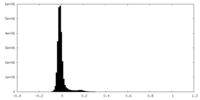
| Density Histograms |
-Half map: O-Layer C14 Local Refinement - Half-B
| File | emd_12707_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | O-Layer C14 Local Refinement - Half-B | ||||||||||||
| Projections & Slices |
| ||||||||||||
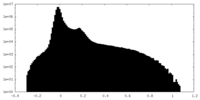
| Density Histograms |
- Sample components
Sample components
-Entire : Type IV secretion system complex
| Entire | Name: Type IV secretion system complex Secretion Secretion |
|---|---|
| Components |
|
-Supramolecule #1: Type IV secretion system complex
| Supramolecule | Name: Type IV secretion system complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: R388 plasmid Escherichia coli (E. coli) / Strain: R388 plasmid |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli)Recombinant plasmid: pBADM11_trwN/virB1-trwE/virB10Strep_rbstrwD/virB11_rbsHistrwB /virD4 |
| Molecular weight | Theoretical: 2.808 MDa |
-Macromolecule #1: TrwE protein
| Macromolecule | Name: TrwE protein / type: protein_or_peptide / ID: 1 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 42.443785 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MFGRKKGDVI DAGAELERAE QERIEGEYGA SELASERRPH TPGARTLLMV LLCVIAVVLV TLSYKAYKVR GVVEDDDAQP QQVVRQVIP GYTPRPIRPE PENVPEPPQP TTSVPAIQPA PVTQPVRPQP TGPREKTPYE LARERMLRSG LTAGSGGGED L PRPQGGDV ...String: MFGRKKGDVI DAGAELERAE QERIEGEYGA SELASERRPH TPGARTLLMV LLCVIAVVLV TLSYKAYKVR GVVEDDDAQP QQVVRQVIP GYTPRPIRPE PENVPEPPQP TTSVPAIQPA PVTQPVRPQP TGPREKTPYE LARERMLRSG LTAGSGGGED L PRPQGGDV PAGGLMGGGG GGGELAEKLQ PMRLSGSSAG RLGNRDMLIT QGTQLDCVLE TRLVTTQPGM TTCHLTRDVY ST SGRVVLL DRGSKVVGFY QGGLRQGQAR IFVQWSRIET PSGVVINLDS PGTGPLGEAG LGGWIDRHFW ERFGGAIMIS LIG DLGDWA SRQGSRQGDN SIQFSNTANG VESAAAEALR NSINIPPTLY KNQGERVNIL VARDLDFSDV YSLESIPTK |
-Macromolecule #2: TrwF protein
| Macromolecule | Name: TrwF protein / type: protein_or_peptide / ID: 2 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 29.749586 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MKKLAIVALL ASLHAVPALA LDVPSSSRYD HRIRYVTYNP ADVVQVDTVL GVATHIMLEE GEQYLTHAFG DSEAYAFARK GRHIFIKPQ AELANTNLIV VTDRRSYKFR LQMRNDRNGA MYELAFRYPD TQARQTREAN ARAAVEAAFE QRVGAYYNLK Y MMSGDKDI ...String: MKKLAIVALL ASLHAVPALA LDVPSSSRYD HRIRYVTYNP ADVVQVDTVL GVATHIMLEE GEQYLTHAFG DSEAYAFARK GRHIFIKPQ AELANTNLIV VTDRRSYKFR LQMRNDRNGA MYELAFRYPD TQARQTREAN ARAAVEAAFE QRVGAYYNLK Y MMSGDKDI APVNAWDDGR FTYFKFSANA DLPSIYFVDA EGNESLVPRT TVGSSNNIIA VHKVNPKWMI RLGNRALAIF NE AYDPNGV PNDTGTASPA VRRVNKGGN |
-Macromolecule #3: TrwH protein
| Macromolecule | Name: TrwH protein / type: protein_or_peptide / ID: 3 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 5.089048 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MKTIIFAILM TGLLSACASA PKPKQPSDFN REPVNKTVPV EIQRGAL |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 57.5 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: CryoSPARC ab-initio |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: OTHER / Details: Stochastic gradient descent (SGD) |
| Final reconstruction | Applied symmetry - Point group: C14 (14 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 2.6 Å / Number images used: 709769 ) / Resolution.type: BY AUTHOR / Resolution: 2.6 Å / Number images used: 709769 |
 Movie
Movie Controller
Controller













 Z
Z Y
Y X
X