+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11940 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
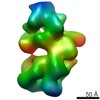
| Title | Ternary complex of Full length Caspase-8 with FADD and FLIPs | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 19.0 Å | |||||||||
 Authors Authors | Fox JL / Ragan TJ / Dinsdale D / Fairall L / Schwabe JWR / Morone N / Cain K / MacFarlane M | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Cryo-EM structural analysis of FADD:Caspase-8 complexes defines the catalytic dimer architecture for co-ordinated control of cell fate. Authors: Joanna L Fox / Michelle A Hughes / Xin Meng / Nikola A Sarnowska / Ian R Powley / Rebekah Jukes-Jones / David Dinsdale / Timothy J Ragan / Louise Fairall / John W R Schwabe / Nobuhiro Morone ...Authors: Joanna L Fox / Michelle A Hughes / Xin Meng / Nikola A Sarnowska / Ian R Powley / Rebekah Jukes-Jones / David Dinsdale / Timothy J Ragan / Louise Fairall / John W R Schwabe / Nobuhiro Morone / Kelvin Cain / Marion MacFarlane /  Abstract: Regulated cell death is essential in development and cellular homeostasis. Multi-protein platforms, including the Death-Inducing Signaling Complex (DISC), co-ordinate cell fate via a core FADD: ...Regulated cell death is essential in development and cellular homeostasis. Multi-protein platforms, including the Death-Inducing Signaling Complex (DISC), co-ordinate cell fate via a core FADD:Caspase-8 complex and its regulatory partners, such as the cell death inhibitor c-FLIP. Here, using electron microscopy, we visualize full-length procaspase-8 in complex with FADD. Our structural analysis now reveals how the FADD-nucleated tandem death effector domain (tDED) helical filament is required to orientate the procaspase-8 catalytic domains, enabling their activation via anti-parallel dimerization. Strikingly, recruitment of c-FLIP into this complex inhibits Caspase-8 activity by altering tDED triple helix architecture, resulting in steric hindrance of the canonical tDED Type I binding site. This prevents both Caspase-8 catalytic domain assembly and tDED helical filament elongation. Our findings reveal how the plasticity, composition and architecture of the core FADD:Caspase-8 complex critically defines life/death decisions not only via the DISC, but across multiple key signaling platforms including TNF complex II, the ripoptosome, and RIPK1/RIPK3 necrosome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11940.map.gz emd_11940.map.gz | 14.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11940-v30.xml emd-11940-v30.xml emd-11940.xml emd-11940.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11940.png emd_11940.png | 69.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11940 http://ftp.pdbj.org/pub/emdb/structures/EMD-11940 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11940 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11940 | HTTPS FTP |
-Validation report
| Summary document |  emd_11940_validation.pdf.gz emd_11940_validation.pdf.gz | 206.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_11940_full_validation.pdf.gz emd_11940_full_validation.pdf.gz | 206 KB | Display | |
| Data in XML |  emd_11940_validation.xml.gz emd_11940_validation.xml.gz | 5.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11940 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11940 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11940 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11940 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11940.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11940.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ternary complex of full-length Caspase-8 with FADD and FLIPs
| Entire | Name: Ternary complex of full-length Caspase-8 with FADD and FLIPs |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of full-length Caspase-8 with FADD and FLIPs
| Supramolecule | Name: Ternary complex of full-length Caspase-8 with FADD and FLIPs type: complex / ID: 1 / Parent: 0 Details: Proteins co-expressed and co-purified with FLAG affinity tag |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pCDNA3 Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pCDNA3 |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Staining | Type: NEGATIVE / Material: Uranyl Acetate Details: Negatively stained EM specimens were prepared following incubation of the sample on the grid overnight at 4 degrees |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI CETA (4k x 4k) / Number grids imaged: 1 / Number real images: 620 / Average exposure time: 1.0 sec. / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 57000 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















