[English] 日本語
 Yorodumi
Yorodumi- EMDB-0320: Cryo-EM informed directed evolution of Nitrilase 4 leads to a cha... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0320 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM informed directed evolution of Nitrilase 4 leads to a change in quaternary structure. | |||||||||
 Map data Map data | Arabidopsis thaliana NITRILASE 4 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NITRILASE / Cyanide detoxification / Beta-cyano-L-alanine hydrolase / Helical filament / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationcyanoalanine nitrilase / 3-cyanoalanine hydratase / cyanoalanine nitrilase activity / 3-cyanoalanine hydratase activity / cyanide metabolic process / nitrilase / indole-3-acetonitrile nitrilase activity / nitrilase activity / detoxification of nitrogen compound / nitrile hydratase activity ...cyanoalanine nitrilase / 3-cyanoalanine hydratase / cyanoalanine nitrilase activity / 3-cyanoalanine hydratase activity / cyanide metabolic process / nitrilase / indole-3-acetonitrile nitrilase activity / nitrilase activity / detoxification of nitrogen compound / nitrile hydratase activity / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
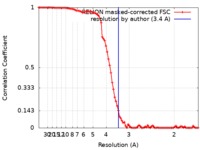
| Method | helical reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Mulelu AE / Woodward JD | |||||||||
| Funding support |  South Africa, South Africa,  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2019 Journal: Commun Biol / Year: 2019Title: Cryo-EM and directed evolution reveal how nitrilase specificity is influenced by its quaternary structure. Authors: Andani E Mulelu / Angela M Kirykowicz / Jeremy D Woodward /  Abstract: Nitrilases are helical enzymes that convert nitriles to acids and/or amides. All plants have a nitrilase 4 homolog specific for ß-cyanoalanine, while in some plants neofunctionalization has produced ...Nitrilases are helical enzymes that convert nitriles to acids and/or amides. All plants have a nitrilase 4 homolog specific for ß-cyanoalanine, while in some plants neofunctionalization has produced nitrilases with altered specificity. Plant nitrilase substrate size and specificity correlate with helical twist, but molecular details of this relationship are lacking. Here we determine, to our knowledge, the first close-to-atomic resolution (3.4 Å) cryo-EM structure of an active helical nitrilase, the nitrilase 4 from . We apply site-saturation mutagenesis directed evolution to three residues (R95, S224, and L169) and generate a mutant with an altered helical twist that accepts substrates not catalyzed by known plant nitrilases. We reveal that a loop between α2 and α3 limits the length of the binding pocket and propose that it shifts position as a function of helical twist. These insights will allow us to start designing nitrilases for chemoenzymatic synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0320.map.gz emd_0320.map.gz | 25.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0320-v30.xml emd-0320-v30.xml emd-0320.xml emd-0320.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0320_fsc.xml emd_0320_fsc.xml | 9 KB | Display |  FSC data file FSC data file |
| Images |  emd_0320.png emd_0320.png | 96.8 KB | ||
| Filedesc metadata |  emd-0320.cif.gz emd-0320.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0320 http://ftp.pdbj.org/pub/emdb/structures/EMD-0320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0320 | HTTPS FTP |
-Validation report
| Summary document |  emd_0320_validation.pdf.gz emd_0320_validation.pdf.gz | 583.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0320_full_validation.pdf.gz emd_0320_full_validation.pdf.gz | 583.3 KB | Display | |
| Data in XML |  emd_0320_validation.xml.gz emd_0320_validation.xml.gz | 10.9 KB | Display | |
| Data in CIF |  emd_0320_validation.cif.gz emd_0320_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0320 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0320 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0320 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0320 | HTTPS FTP |
-Related structure data
| Related structure data |  6i00MC  4406C  4407C 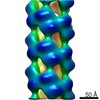 4804C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0320.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0320.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Arabidopsis thaliana NITRILASE 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Active helical nitrilase complex
| Entire | Name: Active helical nitrilase complex |
|---|---|
| Components |
|
-Supramolecule #1: Active helical nitrilase complex
| Supramolecule | Name: Active helical nitrilase complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Bifunctional nitrilase/nitrile hydratase NIT4
| Macromolecule | Name: Bifunctional nitrilase/nitrile hydratase NIT4 / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO / EC number: nitrilase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.766113 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSMQQETSHM TAAPQTNGHQ IFPEIDMSAG DSSSIVRATV VQASTVFYDT PATLDKAERL LSEAAENGSQ LVVFPEAFIG GYPRGSTFE LAIGSRTAKG RDDFRKYHAS AIDVPGPEVE RLALMAKKYK VYLVMGVIER EGYTLYCTVL FFDSQGLFLG K HRKLMPTA ...String: MSMQQETSHM TAAPQTNGHQ IFPEIDMSAG DSSSIVRATV VQASTVFYDT PATLDKAERL LSEAAENGSQ LVVFPEAFIG GYPRGSTFE LAIGSRTAKG RDDFRKYHAS AIDVPGPEVE RLALMAKKYK VYLVMGVIER EGYTLYCTVL FFDSQGLFLG K HRKLMPTA LERCIWGFGD GSTIPVFDTP IGKIGAAICW ENRMPSLRTA MYAKGIEIYC APTADSRETW LASMTHIALE GG CFVLSAN QFCRRKDYPS PPEYMFSGSE ESLTPDSVVC AGGSSIISPL GIVLAGPNYR GEALITADLD LGDIARAKFD FDV VGHYSR PEVFSLNIRE HPRKAVSFKT SKVMEDESVH HHHHH UniProtKB: Bifunctional nitrilase/nitrile hydratase NIT4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 50 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK I Details: The sample (2.5 ul) was applied to the grid and incubated for 30 seconds at 50% humidity before blotting and plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Number real images: 1266 / Average exposure time: 7.0 sec. / Average electron dose: 45.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.75 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Ab initio fitting was performed using Buccaneer, cleaned up with Coot and Phenix Real-Space Refine. |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Target criteria: Cross-correlation coefficient |
| Output model |  PDB-6i00: |
 Movie
Movie Controller
Controller