[English] 日本語
 Yorodumi
Yorodumi- PDB-7nwi: Mammalian pre-termination 80S ribosome with Empty-A site bound by... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7nwi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Mammalian pre-termination 80S ribosome with Empty-A site bound by Blasticidin S | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  RIBOSOME / Inhibitor 80S Termination Complex Blasticidin S Translation NMD RIBOSOME / Inhibitor 80S Termination Complex Blasticidin S Translation NMD | ||||||
| Function / homology |  Function and homology information Function and homology informationFormation of the ternary complex, and subsequently, the 43S complex / Formation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Translation initiation complex formation / Ribosomal scanning and start codon recognition / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit ...Formation of the ternary complex, and subsequently, the 43S complex / Formation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Translation initiation complex formation / Ribosomal scanning and start codon recognition / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit / Major pathway of rRNA processing in the nucleolus and cytosol / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression / SRP-dependent cotranslational protein targeting to membrane / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) /  rough endoplasmic reticulum / rough endoplasmic reticulum /  translation initiation factor binding / translation initiation factor binding /  DNA-(apurinic or apyrimidinic site) lyase / small-subunit processome / cytosolic ribosome / mRNA 5'-UTR binding / DNA-(apurinic or apyrimidinic site) lyase / small-subunit processome / cytosolic ribosome / mRNA 5'-UTR binding /  ribosomal small subunit biogenesis / cytosolic small ribosomal subunit / ribosomal small subunit biogenesis / cytosolic small ribosomal subunit /  heparin binding / small ribosomal subunit / cytoplasmic translation / heparin binding / small ribosomal subunit / cytoplasmic translation /  5S rRNA binding / cytosolic large ribosomal subunit / postsynapse / 5S rRNA binding / cytosolic large ribosomal subunit / postsynapse /  cell differentiation / cell differentiation /  rRNA binding / rRNA binding /  ribosome / structural constituent of ribosome / ribosome / structural constituent of ribosome /  ribonucleoprotein complex / ribonucleoprotein complex /  translation / translation /  mRNA binding / mRNA binding /  synapse / synapse /  nucleolus / negative regulation of apoptotic process / nucleolus / negative regulation of apoptotic process /  endoplasmic reticulum / endoplasmic reticulum /  RNA binding / RNA binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.13 Å cryo EM / Resolution: 3.13 Å | ||||||
 Authors Authors | Powers, K.T. / Yadav, S.K.N. / Bufton, J.C. / Schaffitzel, C. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Blasticidin S inhibits mammalian translation and enhances production of protein encoded by nonsense mRNA. Authors: Kyle T Powers / Flint Stevenson-Jones / Sathish K N Yadav / Beate Amthor / Joshua C Bufton / Ufuk Borucu / Dakang Shen / Jonas P Becker / Daria Lavysh / Matthias W Hentze / Andreas E Kulozik ...Authors: Kyle T Powers / Flint Stevenson-Jones / Sathish K N Yadav / Beate Amthor / Joshua C Bufton / Ufuk Borucu / Dakang Shen / Jonas P Becker / Daria Lavysh / Matthias W Hentze / Andreas E Kulozik / Gabriele Neu-Yilik / Christiane Schaffitzel /   Abstract: Deciphering translation is of paramount importance for the understanding of many diseases, and antibiotics played a pivotal role in this endeavour. Blasticidin S (BlaS) targets translation by binding ...Deciphering translation is of paramount importance for the understanding of many diseases, and antibiotics played a pivotal role in this endeavour. Blasticidin S (BlaS) targets translation by binding to the peptidyl transferase center of the large ribosomal subunit. Using biochemical, structural and cellular approaches, we show here that BlaS inhibits both translation elongation and termination in Mammalia. Bound to mammalian terminating ribosomes, BlaS distorts the 3'CCA tail of the P-site tRNA to a larger extent than previously reported for bacterial ribosomes, thus delaying both, peptide bond formation and peptidyl-tRNA hydrolysis. While BlaS does not inhibit stop codon recognition by the eukaryotic release factor 1 (eRF1), it interferes with eRF1's accommodation into the peptidyl transferase center and subsequent peptide release. In human cells, BlaS inhibits nonsense-mediated mRNA decay and, at subinhibitory concentrations, modulates translation dynamics at premature termination codons leading to enhanced protein production. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7nwi.cif.gz 7nwi.cif.gz | 7.8 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7nwi.ent.gz pdb7nwi.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7nwi.json.gz 7nwi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi https://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi ftp://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi ftp://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  12633MC  7nwgC  7nwhC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+Protein , 32 types, 32 molecules ABFHLOPQSTVXacdefhkorstEEKKOOQQRRTTUUVVff
-60S ribosomal protein ... , 14 types, 14 molecules CDEGIRUWZbgimn
| #3: Protein |  Mass: 47727.559 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
|---|---|
| #4: Protein |  Mass: 34481.828 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SYJ6 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SYJ6 |
| #5: Protein |  Mass: 33055.297 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SKF7 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SKF7 |
| #7: Protein |  Mass: 27480.555 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1STW0 Oryctolagus cuniculus (rabbit) / References: UniProt: G1STW0 |
| #9: Protein |  / Ribosomal protein L10 (Predicted) / Ribosomal protein L10 (Predicted)Mass: 24643.057 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: B7NZQ2 Oryctolagus cuniculus (rabbit) / References: UniProt: B7NZQ2 |
| #17: Protein |  Mass: 23535.281 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: Q3T0W9 Oryctolagus cuniculus (rabbit) / References: UniProt: Q3T0W9 |
| #20: Protein |  Mass: 14784.962 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: Q4R5I3 Oryctolagus cuniculus (rabbit) / References: UniProt: Q4R5I3 |
| #22: Protein |  Ribosome RibosomeMass: 15538.256 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: A0A061I3X8 Oryctolagus cuniculus (rabbit) / References: UniProt: A0A061I3X8 |
| #25: Protein |  Mass: 15704.635 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TXF6 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TXF6 |
| #27: Protein |  Mass: 26708.707 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #32: Protein |  Mass: 14210.088 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1U945 Oryctolagus cuniculus (rabbit) / References: UniProt: G1U945 |
| #34: Protein |  Mass: 13546.292 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #38: Protein |  / CEP52 / Ubiquitin / Ubiquitin A-52 residue ribosomal protein fusion product 1 / Ubiquitin-60S ...CEP52 / Ubiquitin / Ubiquitin A-52 residue ribosomal protein fusion product 1 / Ubiquitin-60S ribosomal protein L40 / CEP52 / Ubiquitin / Ubiquitin A-52 residue ribosomal protein fusion product 1 / Ubiquitin-60S ...CEP52 / Ubiquitin / Ubiquitin A-52 residue ribosomal protein fusion product 1 / Ubiquitin-60S ribosomal protein L40Mass: 14695.310 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: A0A6P4TG29 Oryctolagus cuniculus (rabbit) / References: UniProt: A0A6P4TG29 |
| #39: Protein/peptide |  Mass: 3213.075 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: A0A087WNH4 Oryctolagus cuniculus (rabbit) / References: UniProt: A0A087WNH4 |
-Ribosomal protein ... , 15 types, 15 molecules JMNYjlpFFHHNNSSWWXXddgg
| #10: Protein |  Mass: 20670.904 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
|---|---|
| #12: Protein |  Mass: 23870.549 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SZ12 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SZ12 |
| #13: Protein |  Mass: 24076.088 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1T0C1 Oryctolagus cuniculus (rabbit) / References: UniProt: G1T0C1 |
| #24: Protein |  Mass: 15891.787 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SQH0 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SQH0 |
| #35: Protein |  Mass: 11111.032 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: U3KPD5 Oryctolagus cuniculus (rabbit) / References: UniProt: U3KPD5 |
| #37: Protein/peptide |  Mass: 6295.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SYU7 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SYU7 |
| #41: Protein |  Mass: 10168.153 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SY53 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SY53 |
| #50: Protein |  Mass: 23044.650 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TFM5 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TFM5 |
| #52: Protein |  Mass: 21716.387 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SVB0 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SVB0 |
| #58: Protein |  Mass: 17128.191 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SP51 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SP51 |
| #63: Protein |  Mass: 16170.774 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TPG3 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TPG3 |
| #67: Protein |  Ribosome RibosomeMass: 15778.584 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #68: Protein |  Mass: 15626.392 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SZ47 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SZ47 |
| #74: Protein |  Mass: 6364.426 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1U7M4 Oryctolagus cuniculus (rabbit) / References: UniProt: G1U7M4 |
| #77: Protein |  Mass: 34669.113 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SJB4 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SJB4 |
-40S ribosomal protein ... , 16 types, 16 molecules AABBCCDDGGIIJJLLMMPPYYZZaabbccee
| #45: Protein |  / 37 kDa laminin receptor precursor / 37LRP / 37/67 kDa laminin receptor / LRP/LR / 67 kDa laminin ...37 kDa laminin receptor precursor / 37LRP / 37/67 kDa laminin receptor / LRP/LR / 67 kDa laminin receptor / 67LR / Laminin receptor 1 / LamR / Laminin-binding protein precursor p40 / LBP/p40 / 37 kDa laminin receptor precursor / 37LRP / 37/67 kDa laminin receptor / LRP/LR / 67 kDa laminin ...37 kDa laminin receptor precursor / 37LRP / 37/67 kDa laminin receptor / LRP/LR / 67 kDa laminin receptor / 67LR / Laminin receptor 1 / LamR / Laminin-binding protein precursor p40 / LBP/p40Mass: 32927.988 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TLT8 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TLT8 |
|---|---|
| #46: Protein |  / Protein TU-11 / Protein TU-11Mass: 29942.010 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: P97351 Oryctolagus cuniculus (rabbit) / References: UniProt: P97351 |
| #47: Protein |  Mass: 27956.623 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: O55214 Oryctolagus cuniculus (rabbit) / References: UniProt: O55214 |
| #48: Protein |  Mass: 31146.607 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit)References: UniProt: G1TNM3,  DNA-(apurinic or apyrimidinic site) lyase DNA-(apurinic or apyrimidinic site) lyase |
| #51: Protein |  Mass: 30070.432 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: A0A6G1APX9 Oryctolagus cuniculus (rabbit) / References: UniProt: A0A6G1APX9 |
| #53: Protein |  Mass: 24003.012 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TJW1 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TJW1 |
| #54: Protein |  Mass: 22655.590 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: A0A1S3APZ1 Oryctolagus cuniculus (rabbit) / References: UniProt: A0A1S3APZ1 |
| #56: Protein |  Mass: 18468.826 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TRM4 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TRM4 |
| #57: Protein |  Mass: 13766.122 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1SFR8 Oryctolagus cuniculus (rabbit) / References: UniProt: G1SFR8 |
| #60: Protein |  Mass: 17049.182 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1U0Q2 Oryctolagus cuniculus (rabbit) / References: UniProt: G1U0Q2 |
| #69: Protein |  Mass: 17007.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #70: Protein |  Mass: 13581.994 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TDB3 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TDB3 |
| #71: Protein |  Mass: 13147.561 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: T0M5X4 Oryctolagus cuniculus (rabbit) / References: UniProt: T0M5X4 |
| #72: Protein |  Mass: 9480.186 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TZ76 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TZ76 |
| #73: Protein |  Mass: 7776.891 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1TIB4 Oryctolagus cuniculus (rabbit) / References: UniProt: G1TIB4 |
| #75: Protein |  Mass: 14498.884 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: UniProt: G1T8A2 Oryctolagus cuniculus (rabbit) / References: UniProt: G1T8A2 |
-Protein/peptide , 1 types, 1 molecules 1
| #78: Protein/peptide | Mass: 1788.032 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
|---|
-RNA chain , 6 types, 6 molecules 25789K
| #79: RNA chain | Mass: 24436.551 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
|---|---|
| #80: RNA chain | Mass: 1226739.500 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #81: RNA chain |  Mass: 38691.914 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: Oryctolagus cuniculus (rabbit) / References:  GenBank: 4CXE_4 GenBank: 4CXE_4 |
| #82: RNA chain |  Mass: 50143.648 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) / References: Oryctolagus cuniculus (rabbit) / References:  GenBank: 4CXE_3 GenBank: 4CXE_3 |
| #83: RNA chain |  Mass: 573221.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| #84: RNA chain |  Messenger RNA Messenger RNAMass: 3225.980 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
-Non-polymers , 3 types, 216 molecules 

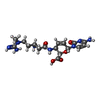


| #85: Chemical | ChemComp-MG / #86: Chemical | ChemComp-ZN / #87: Chemical | ChemComp-BLS / |  Blasticidin S Blasticidin S |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Mammalian pre-termination 80S ribosome with Empty-A site bound by Blasticidin S Type: RIBOSOME Details: Rabbit reticulocyte lysate derived (in vitro transcription-FLAG affinity purified) ribosomal complex. Entity ID: #1-#2, #4-#8, #10-#14, #16-#20, #22-#31, #33-#34, #36-#37, #39, #42-#79 Source: NATURAL |
|---|---|
| Molecular weight | Value: 3.8 MDa / Experimental value: NO |
| Source (natural) | Organism:   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| Buffer solution | pH: 7.4 Details: 50 mM HEPES pH 7.4 100 mM KOAc 5 mM Mg(Oac)2 1mM DTT |
| Specimen | Conc.: 0.63 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES / Details: 165nM (OD260nm~10.5) : YES / Details: 165nM (OD260nm~10.5) |
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/2 |
Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 70 % / Chamber temperature: 288.15 K Details: 30s sample incubation on grid followed by 1.1s blotting time. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TALOS ARCTICA Details: Collected in super-resolution mode (0.675A pixel size) |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Calibrated magnification: 79000 X / Calibrated defocus min: 400 nm / Calibrated defocus max: 2000 nm / Cs Bright-field microscopy / Calibrated magnification: 79000 X / Calibrated defocus min: 400 nm / Calibrated defocus max: 2000 nm / Cs : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 8 sec. / Electron dose: 41.92 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 3500 |
| Image scans | Movie frames/image: 40 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 730463 | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.13 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 103842 / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 3JAH | ||||||||||||||||||||||||||||||||
| Refinement | Stereochemistry target values: GeoStd + Monomer Library | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj



































