[English] 日本語
 Yorodumi
Yorodumi- PDB-7a49: Crystal structure of human protein kinase CK2alpha (CSNK2A1 gene ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7a49 | ||||||
|---|---|---|---|---|---|---|---|
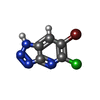
| Title | Crystal structure of human protein kinase CK2alpha (CSNK2A1 gene product) in complex with the ATP-competitive inhibitor 6-bromo-5-chloro-1H-triazolo[4,5-b]pyridine | ||||||
 Components Components | Casein kinase II subunit alpha Casein kinase 2 Casein kinase 2 | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  protein kinase CK2 / protein kinase CK2 /  casein kinase 2 / ATP-competitive inhibitor casein kinase 2 / ATP-competitive inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of chromosome separation / positive regulation of aggrephagy / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes /  protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Maturation of hRSV A proteins / Receptor Mediated Mitophagy / Sin3-type complex / Synthesis of PC ...regulation of chromosome separation / positive regulation of aggrephagy / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes / protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Maturation of hRSV A proteins / Receptor Mediated Mitophagy / Sin3-type complex / Synthesis of PC ...regulation of chromosome separation / positive regulation of aggrephagy / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes /  protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Maturation of hRSV A proteins / Receptor Mediated Mitophagy / Sin3-type complex / Synthesis of PC / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / negative regulation of apoptotic signaling pathway / positive regulation of Wnt signaling pathway / negative regulation of double-strand break repair via homologous recombination / chaperone-mediated protein folding / negative regulation of ubiquitin-dependent protein catabolic process / Signal transduction by L1 / peptidyl-threonine phosphorylation / protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Maturation of hRSV A proteins / Receptor Mediated Mitophagy / Sin3-type complex / Synthesis of PC / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / negative regulation of apoptotic signaling pathway / positive regulation of Wnt signaling pathway / negative regulation of double-strand break repair via homologous recombination / chaperone-mediated protein folding / negative regulation of ubiquitin-dependent protein catabolic process / Signal transduction by L1 / peptidyl-threonine phosphorylation /  Hsp90 protein binding / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / PML body / Hsp90 protein binding / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / PML body /  Wnt signaling pathway / Regulation of PTEN stability and activity / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / positive regulation of protein catabolic process / KEAP1-NFE2L2 pathway / double-strand break repair / rhythmic process / Wnt signaling pathway / Regulation of PTEN stability and activity / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / positive regulation of protein catabolic process / KEAP1-NFE2L2 pathway / double-strand break repair / rhythmic process /  kinase activity / positive regulation of cell growth / peptidyl-serine phosphorylation / Regulation of TP53 Activity through Phosphorylation / negative regulation of translation / protein stabilization / kinase activity / positive regulation of cell growth / peptidyl-serine phosphorylation / Regulation of TP53 Activity through Phosphorylation / negative regulation of translation / protein stabilization /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  regulation of cell cycle / regulation of cell cycle /  cell cycle / cell cycle /  protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / DNA damage response / positive regulation of cell population proliferation / protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / DNA damage response / positive regulation of cell population proliferation /  signal transduction / signal transduction /  nucleoplasm / nucleoplasm /  ATP binding / identical protein binding / ATP binding / identical protein binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.03 Å MOLECULAR REPLACEMENT / Resolution: 2.03 Å | ||||||
 Authors Authors | Niefind, K. / Lindenblatt, D. / Toelzer, C. / Bretner, M. / Chojnacki, K. / Wielechowska, M. / Winska, P. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Bioorg.Chem. / Year: 2021 Journal: Bioorg.Chem. / Year: 2021Title: Synthesis, biological properties and structural study of new halogenated azolo[4,5-b]pyridines as inhibitors of CK2 kinase. Authors: Chojnacki, K. / Lindenblatt, D. / Winska, P. / Wielechowska, M. / Toelzer, C. / Niefind, K. / Bretner, M. #1:  Journal: Bioorg. Chem. / Year: 2018 Journal: Bioorg. Chem. / Year: 2018Title: Biological properties and structural study of new aminoalkyl derivatives of benzimidazole and benzotriazole, dual inhibitors of CK2 and PIM1 kinases. Authors: Chojnacki, K. / Winska, P. / Wielechowska, M. / Lukowska-Chojnacka, E. / Toelzer, C. / Niefind, K. / Bretner, M. #2:  Journal: ACS Omega / Year: 2019 Journal: ACS Omega / Year: 2019Title: Diacritic Binding of an Indenoindole Inhibitor by CK2alpha Paralogs Explored by a Reliable Path to Atomic Resolution CK2alpha' Structures. Authors: Lindenblatt, D. / Nickelsen, A. / Applegate, V.M. / Hochscherf, J. / Witulski, B. / Bouaziz, Z. / Marminon, C. / Bretner, M. / Le Borgne, M. / Jose, J. / Niefind, K. #3:  Journal: J. Med. Chem. / Year: 2020 Journal: J. Med. Chem. / Year: 2020Title: Structural and Mechanistic Basis of the Inhibitory Potency of Selected 2-Aminothiazole Compounds on Protein Kinase CK2. Authors: Lindenblatt, D. / Nickelsen, A. / Applegate, V.M. / Jose, J. / Niefind, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7a49.cif.gz 7a49.cif.gz | 356.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7a49.ent.gz pdb7a49.ent.gz | 244.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7a49.json.gz 7a49.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/a4/7a49 https://data.pdbj.org/pub/pdb/validation_reports/a4/7a49 ftp://data.pdbj.org/pub/pdb/validation_reports/a4/7a49 ftp://data.pdbj.org/pub/pdb/validation_reports/a4/7a49 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7a1bC  7a1zC  7a22C  7a2hC  7a4bC  7a4cC  2pvrS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS oper: (Code: givenMatrix: (0.00907483492286, -0.997387409777, 0.0716659067393), (0.999860108723, 0.00804359631376, -0.0146650448974), (0.0140502795212, 0.0717889641657, 0.997320878288)Vector: -64. ...NCS oper: (Code: given Matrix: (0.00907483492286, -0.997387409777, 0.0716659067393), Vector  : : |
- Components
Components
| #1: Protein |  Casein kinase 2 / CK II alpha Casein kinase 2 / CK II alphaMass: 40066.742 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: CSNK2A1, CK2A1 / Production host: Homo sapiens (human) / Gene: CSNK2A1, CK2A1 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P68400,  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase#2: Chemical | #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate#4: Water | ChemComp-HOH / |  Water WaterHas ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.16 Å3/Da / Density % sol: 61.11 % |
|---|---|
Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, sitting drop Details: 1 MIKROLITER OF THE CK2ALPHA/INHIBITOR MIXTURE (COMPOSITION: 7 MG/ML CK2ALPHA ENZYME, 1 MILLIMOLAR INHIBITOR, 10 % DIMETHYL SULFOXIDE, 450 MM NACL, 22.5 MM TRIS/HCL, PH 8.5) WAS MIXED WITH 1 ...Details: 1 MIKROLITER OF THE CK2ALPHA/INHIBITOR MIXTURE (COMPOSITION: 7 MG/ML CK2ALPHA ENZYME, 1 MILLIMOLAR INHIBITOR, 10 % DIMETHYL SULFOXIDE, 450 MM NACL, 22.5 MM TRIS/HCL, PH 8.5) WAS MIXED WITH 1 MIKROLITER RESERVOIR SOLUTION (COMPOSITION: 25 % PEG3350, 0.2 M AMMONIUM SULPHATE, 0.1 M BIS-TRIS BUFFER, PH 5.5) FOLLOWED BY VAPOUR DIFFUSION EQUILIBRATION AGAINST THE RESERVOIR SOLUTION. |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.976251 Å / Beamline: ID29 / Wavelength: 0.976251 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Dec 13, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.976251 Å / Relative weight: 1 : 0.976251 Å / Relative weight: 1 |
| Reflection | Resolution: 2.028→89.135 Å / Num. obs: 60321 / % possible obs: 90 % / Redundancy: 13.1 % / Biso Wilson estimate: 49.51 Å2 / CC1/2: 1 / Rmerge(I) obs: 0.077 / Rpim(I) all: 0.022 / Rrim(I) all: 0.08 / Rsym value: 0.077 / Net I/σ(I): 17.9 |
| Reflection shell | Resolution: 2.028→2.136 Å / Redundancy: 12.7 % / Rmerge(I) obs: 1.827 / Mean I/σ(I) obs: 1.3 / Num. unique obs: 3018 / CC1/2: 0.563 / Rpim(I) all: 0.525 / Rrim(I) all: 1.892 / Rsym value: 1.827 / % possible all: 31.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2PVR Resolution: 2.03→89.13 Å / SU ML: 0.2677 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 25.2834 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 56.21 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.03→89.13 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Type: Torsion NCS / Rms dev position: 1.15500035223 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj