+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6td4 | ||||||
|---|---|---|---|---|---|---|---|
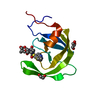
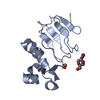
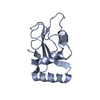
| Title | IRF4 DNA-binding domain surface entropy mutant apo structure | ||||||
 Components Components | Interferon regulatory factor 4 Interferon regulatory factors Interferon regulatory factors | ||||||
 Keywords Keywords |  DNA BINDING PROTEIN / DNA BINDING PROTEIN /  DNA BINDING DOMAIN / SURFACE ENTROPY MUTANT / APO DNA BINDING DOMAIN / SURFACE ENTROPY MUTANT / APO | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of toll-like receptor signaling pathway / T-helper 17 cell lineage commitment / regulation of T-helper cell differentiation / myeloid dendritic cell differentiation / positive regulation of interleukin-13 production /  immune system process / defense response to protozoan / positive regulation of interleukin-10 production / positive regulation of interleukin-4 production / positive regulation of interleukin-2 production ...negative regulation of toll-like receptor signaling pathway / T-helper 17 cell lineage commitment / regulation of T-helper cell differentiation / myeloid dendritic cell differentiation / positive regulation of interleukin-13 production / immune system process / defense response to protozoan / positive regulation of interleukin-10 production / positive regulation of interleukin-4 production / positive regulation of interleukin-2 production ...negative regulation of toll-like receptor signaling pathway / T-helper 17 cell lineage commitment / regulation of T-helper cell differentiation / myeloid dendritic cell differentiation / positive regulation of interleukin-13 production /  immune system process / defense response to protozoan / positive regulation of interleukin-10 production / positive regulation of interleukin-4 production / positive regulation of interleukin-2 production / immune system process / defense response to protozoan / positive regulation of interleukin-10 production / positive regulation of interleukin-4 production / positive regulation of interleukin-2 production /  T cell activation / Interferon gamma signaling / Interferon alpha/beta signaling / T cell activation / Interferon gamma signaling / Interferon alpha/beta signaling /  nucleosome / sequence-specific double-stranded DNA binding / positive regulation of cold-induced thermogenesis / DNA-binding transcription activator activity, RNA polymerase II-specific / Interleukin-4 and Interleukin-13 signaling / sequence-specific DNA binding / nucleosome / sequence-specific double-stranded DNA binding / positive regulation of cold-induced thermogenesis / DNA-binding transcription activator activity, RNA polymerase II-specific / Interleukin-4 and Interleukin-13 signaling / sequence-specific DNA binding /  transcription coactivator activity / DNA-binding transcription factor activity, RNA polymerase II-specific / transcription coactivator activity / DNA-binding transcription factor activity, RNA polymerase II-specific /  chromatin remodeling / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / chromatin remodeling / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity /  chromatin / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II / chromatin / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II /  nucleoplasm / nucleoplasm /  membrane / membrane /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.71 Å MOLECULAR REPLACEMENT / Resolution: 1.71 Å | ||||||
 Authors Authors | Tucker, J.A. / Martin, M.P. / Wang, L.Z. / Jennings, C. / Heath, R. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cancer-associated mutations in the IRF4 DNA-binding domain confer no disadvantage in DNA-binding affinity and may increase transcriptional activity Authors: Tatum, N.J. / Scott, R. / Doody, G.M. / Hickson, I. / Jennings, C. / Martin, M.P. / Tooze, R.M. / Tucker, J.A. / Wittner, A. / Wang, L.Z. / Wright, E.K. / Wedge, S.R. / Noble, M.E.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6td4.cif.gz 6td4.cif.gz | 76 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6td4.ent.gz pdb6td4.ent.gz | 46.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6td4.json.gz 6td4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/td/6td4 https://data.pdbj.org/pub/pdb/validation_reports/td/6td4 ftp://data.pdbj.org/pub/pdb/validation_reports/td/6td4 ftp://data.pdbj.org/pub/pdb/validation_reports/td/6td4 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein |  Interferon regulatory factors / IRF-4 / Lymphocyte-specific interferon regulatory factor / LSIRF / Multiple myeloma oncogene 1 / NF-EM5 Interferon regulatory factors / IRF-4 / Lymphocyte-specific interferon regulatory factor / LSIRF / Multiple myeloma oncogene 1 / NF-EM5Mass: 13938.777 Da / Num. of mol.: 1 / Mutation: E45A E46A K47A C99A Source method: isolated from a genetically manipulated source Details: Additional residues at N- and C-termini from cloning Source: (gene. exp.)   Homo sapiens (human) / Gene: IRF4, MUM1 / Production host: Homo sapiens (human) / Gene: IRF4, MUM1 / Production host:   Escherichia coli BL21(DE3) (bacteria) / Variant (production host): Rosetta (DE3) pLysS / References: UniProt: Q15306 Escherichia coli BL21(DE3) (bacteria) / Variant (production host): Rosetta (DE3) pLysS / References: UniProt: Q15306 |
|---|---|
| #2: Chemical | ChemComp-CL /  Chloride Chloride |
| #3: Water | ChemComp-HOH /  Water Water |
| Has ligand of interest | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.83 Å3/Da / Density % sol: 56.49 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7.5 / Details: 25 % PEG 3350, 0.1 M HEPES pH 7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.92 Å / Beamline: I04-1 / Wavelength: 0.92 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Jul 14, 2016 |
| Radiation | Monochromator: single bounce monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.92 Å / Relative weight: 1 : 0.92 Å / Relative weight: 1 |
| Reflection | Resolution: 1.71→39.28 Å / Num. obs: 17568 / % possible obs: 100 % / Redundancy: 6.5 % / Biso Wilson estimate: 33.56 Å2 / CC1/2: 0.994 / Rrim(I) all: 0.041 / Net I/σ(I): 16.9 |
| Reflection shell | Resolution: 1.71→1.78 Å / Redundancy: 5.7 % / Mean I/σ(I) obs: 1.7 / Num. unique obs: 2774 / CC1/2: 0.839 / Rrim(I) all: 1.12 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: personal communication Resolution: 1.71→39.28 Å / SU ML: 0.2108 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 37.9394 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 52.64 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.71→39.28 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj




