[English] 日本語
 Yorodumi
Yorodumi- PDB-5e2l: 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase from Mycobac... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5e2l | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase from Mycobacterium tuberculosis in complex with D-phenylalanine | |||||||||
 Components Components | 3-deoxy-D-arabinoheptulosonate-7-phosphate synthase | |||||||||
 Keywords Keywords |  TRANSFERASE / 3-Deoxy-7-Phosphoheptulosonate Synthase / TRANSFERASE / 3-Deoxy-7-Phosphoheptulosonate Synthase /  allosteric regulation / allosteric regulation /  allosteric site / allosteric site /  amino acid amino acid | |||||||||
| Function / homology |  Function and homology information Function and homology information 3-deoxy-7-phosphoheptulonate synthase / 3-deoxy-7-phosphoheptulonate synthase /  3-deoxy-7-phosphoheptulonate synthase activity / Chorismate via Shikimate Pathway / chorismate biosynthetic process / aromatic amino acid family biosynthetic process / amino acid biosynthetic process / peptidoglycan-based cell wall / protein homooligomerization / manganese ion binding / 3-deoxy-7-phosphoheptulonate synthase activity / Chorismate via Shikimate Pathway / chorismate biosynthetic process / aromatic amino acid family biosynthetic process / amino acid biosynthetic process / peptidoglycan-based cell wall / protein homooligomerization / manganese ion binding /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Mycobacterium tuberculosis (bacteria) Mycobacterium tuberculosis (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | |||||||||
 Authors Authors | Reichau, S. / Jiao, W. / Parker, E.J. | |||||||||
| Funding support |  New Zealand, 2items New Zealand, 2items
| |||||||||
 Citation Citation |  Journal: Plos One / Year: 2016 Journal: Plos One / Year: 2016Title: Probing the Sophisticated Synergistic Allosteric Regulation of Aromatic Amino Acid Biosynthesis in Mycobacterium tuberculosis Using -Amino Acids. Authors: Reichau, S. / Blackmore, N.J. / Jiao, W. / Parker, E.J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5e2l.cif.gz 5e2l.cif.gz | 357.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5e2l.ent.gz pdb5e2l.ent.gz | 292.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5e2l.json.gz 5e2l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e2/5e2l https://data.pdbj.org/pub/pdb/validation_reports/e2/5e2l ftp://data.pdbj.org/pub/pdb/validation_reports/e2/5e2l ftp://data.pdbj.org/pub/pdb/validation_reports/e2/5e2l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5e40C  5e4nC  5e5gC  5e7zC  3nv8S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 50828.395 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mycobacterium tuberculosis (bacteria) Mycobacterium tuberculosis (bacteria)Gene: aroG_1, ERS024751_03564, ERS094182_00944, ERS124362_02783 Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: A0A0E8NFD1, UniProt: O53512*PLUS,  3-deoxy-7-phosphoheptulonate synthase 3-deoxy-7-phosphoheptulonate synthase |
|---|
-Non-polymers , 6 types, 98 molecules 



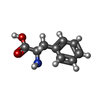






| #2: Chemical | ChemComp-SO4 /  Sulfate Sulfate#3: Chemical | #4: Chemical | ChemComp-CL /  Chloride Chloride#5: Chemical |  Glycerol Glycerol#6: Chemical |  Phenylalanine Phenylalanine#7: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.95 Å3/Da / Density % sol: 68.88 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1 M TRIS-HCl, pH 7.5, 1.5M ammonium sulfate, 12% v/v glycerol. Crystals were soaked in the same solution with an additional 10% v/v glycerol and 2.5 mM D-phenylalanine |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX1 / Wavelength: 0.97948 Å / Beamline: MX1 / Wavelength: 0.97948 Å | |||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 210r / Detector: CCD / Date: Sep 26, 2012 | |||||||||||||||||||||||||||
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.97948 Å / Relative weight: 1 : 0.97948 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection twin |
| |||||||||||||||||||||||||||
| Reflection | Resolution: 2.5→49 Å / Num. obs: 55365 / % possible obs: 100 % / Redundancy: 11.2 % / CC1/2: 0.993 / Rmerge(I) obs: 0.19 / Rpim(I) all: 0.059 / Net I/σ(I): 12 / Num. measured all: 620384 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: 0
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 3NV8 Resolution: 2.5→49 Å / Cor.coef. Fo:Fc: 0.948 / Cor.coef. Fo:Fc free: 0.93 / WRfactor Rfree: 0.1462 / WRfactor Rwork: 0.131 / FOM work R set: 0.8967 / SU B: 7.197 / SU ML: 0.083 / SU R Cruickshank DPI: 0.0465 / SU Rfree: 0.0347 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.046 / ESU R Free: 0.035 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 109.88 Å2 / Biso mean: 35.628 Å2 / Biso min: 17.26 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.5→49 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.565 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj





