[English] 日本語
 Yorodumi
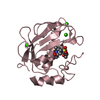
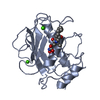
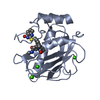
Yorodumi- PDB-3qx8: Crystal structure of MID domain from hAGO2 in complex with m7GpppG -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3qx8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of MID domain from hAGO2 in complex with m7GpppG | ||||||
 Components Components | Protein argonaute-2 | ||||||
 Keywords Keywords |  RNA BINDING PROTEIN / Rossmann-like folde / RNA BINDING PROTEIN / Rossmann-like folde /  RNA binding / RNA binding /  small RNA small RNA | ||||||
| Function / homology |  Function and homology information Function and homology information: / endoribonuclease activity, cleaving miRNA-paired mRNA / endoribonuclease activity, cleaving siRNA-paired mRNA / siRNA-mediated gene silencing by mRNA destabilization / miRNA-mediated gene silencing by mRNA destabilization / Regulation of CDH11 mRNA translation by microRNAs / Regulation of NPAS4 mRNA translation / Post-transcriptional silencing by small RNAs / Competing endogenous RNAs (ceRNAs) regulate PTEN translation / Regulation of PTEN mRNA translation ...: / endoribonuclease activity, cleaving miRNA-paired mRNA / endoribonuclease activity, cleaving siRNA-paired mRNA / siRNA-mediated gene silencing by mRNA destabilization / miRNA-mediated gene silencing by mRNA destabilization / Regulation of CDH11 mRNA translation by microRNAs / Regulation of NPAS4 mRNA translation / Post-transcriptional silencing by small RNAs / Competing endogenous RNAs (ceRNAs) regulate PTEN translation / Regulation of PTEN mRNA translation / negative regulation of amyloid precursor protein biosynthetic process / Small interfering RNA (siRNA) biogenesis / Transcriptional Regulation by MECP2 / positive regulation of trophoblast cell migration / RNA secondary structure unwinding / miRNA metabolic process / RISC-loading complex / mRNA cap binding / regulatory ncRNA-mediated post-transcriptional gene silencing /  : / RISC complex assembly / mRNA 3'-UTR AU-rich region binding / miRNA processing / pre-miRNA processing / miRNA-mediated gene silencing by inhibition of translation / siRNA processing / RNA 7-methylguanosine cap binding / siRNA binding / M-decay: degradation of maternal mRNAs by maternally stored factors / regulatory ncRNA-mediated gene silencing / RISC complex / Regulation of RUNX1 Expression and Activity / MicroRNA (miRNA) biogenesis / miRNA binding / positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / RNA polymerase II complex binding / Regulation of MECP2 expression and activity / core promoter sequence-specific DNA binding / negative regulation of translational initiation / RNA endonuclease activity / NR1H3 & NR1H2 regulate gene expression linked to cholesterol transport and efflux / : / RISC complex assembly / mRNA 3'-UTR AU-rich region binding / miRNA processing / pre-miRNA processing / miRNA-mediated gene silencing by inhibition of translation / siRNA processing / RNA 7-methylguanosine cap binding / siRNA binding / M-decay: degradation of maternal mRNAs by maternally stored factors / regulatory ncRNA-mediated gene silencing / RISC complex / Regulation of RUNX1 Expression and Activity / MicroRNA (miRNA) biogenesis / miRNA binding / positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / RNA polymerase II complex binding / Regulation of MECP2 expression and activity / core promoter sequence-specific DNA binding / negative regulation of translational initiation / RNA endonuclease activity / NR1H3 & NR1H2 regulate gene expression linked to cholesterol transport and efflux /  translation initiation factor activity / post-embryonic development / positive regulation of translation / translation initiation factor activity / post-embryonic development / positive regulation of translation /  P-body / TP53 Regulates Metabolic Genes / Transcriptional regulation by small RNAs / MAPK6/MAPK4 signaling / cytoplasmic ribonucleoprotein granule / Pre-NOTCH Transcription and Translation / positive regulation of angiogenesis / P-body / TP53 Regulates Metabolic Genes / Transcriptional regulation by small RNAs / MAPK6/MAPK4 signaling / cytoplasmic ribonucleoprotein granule / Pre-NOTCH Transcription and Translation / positive regulation of angiogenesis /  double-stranded RNA binding / Ca2+ pathway / Estrogen-dependent gene expression / double-stranded RNA binding / Ca2+ pathway / Estrogen-dependent gene expression /  single-stranded RNA binding / single-stranded RNA binding /  translation / translation /  dendrite / positive regulation of transcription by RNA polymerase II / dendrite / positive regulation of transcription by RNA polymerase II /  RNA binding / extracellular exosome / RNA binding / extracellular exosome /  nucleoplasm / nucleoplasm /  membrane / membrane /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FOURIER SYNTHESIS / Resolution: 2.3 Å FOURIER SYNTHESIS / Resolution: 2.3 Å | ||||||
 Authors Authors | Frank, F. / Fabian, M.R. / Stepinski, J. / Jemielity, J. / Darzynkiewicz, E. / Sonenberg, N. / Nagar, B. | ||||||
 Citation Citation |  Journal: Embo Rep. / Year: 2011 Journal: Embo Rep. / Year: 2011Title: Structural analysis of 5'-mRNA-cap interactions with the human AGO2 MID domain. Authors: Frank, F. / Fabian, M.R. / Stepinski, J. / Jemielity, J. / Darzynkiewicz, E. / Sonenberg, N. / Nagar, B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3qx8.cif.gz 3qx8.cif.gz | 93.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3qx8.ent.gz pdb3qx8.ent.gz | 71.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3qx8.json.gz 3qx8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qx/3qx8 https://data.pdbj.org/pub/pdb/validation_reports/qx/3qx8 ftp://data.pdbj.org/pub/pdb/validation_reports/qx/3qx8 ftp://data.pdbj.org/pub/pdb/validation_reports/qx/3qx8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3qx9C  3lucS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 15325.955 Da / Num. of mol.: 3 / Fragment: unp residues 439-575 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: EIF2C2, AGO2 / Production host: Homo sapiens (human) / Gene: EIF2C2, AGO2 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9UKV8 Escherichia coli (E. coli) / References: UniProt: Q9UKV8#2: Chemical | #3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.93 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 0.1 M imidazole, 0.2 M NaCl, 0.46 M NaH2PO4, 1.84 M K2HPO4, pH 8.0, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 200 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 |
| Detector | Type: RIGAKU SATURN 944+ / Detector: CCD / Date: Mar 23, 2010 |
| Radiation | Monochromator: Varimax HF / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→30 Å / Num. all: 20712 / Num. obs: 17438 / % possible obs: 84.2 % |
- Processing
Processing
| Software |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB entry 3LUC Resolution: 2.3→30 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→30 Å
|
 Movie
Movie Controller
Controller









 PDBj
PDBj
















