[English] 日本語
 Yorodumi
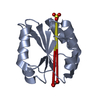
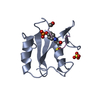
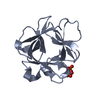
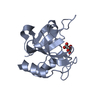
Yorodumi- PDB-1dzd: High resolution structure of acidic fibroblast growth factor (27-... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1dzd | ||||||
|---|---|---|---|---|---|---|---|
| Title | High resolution structure of acidic fibroblast growth factor (27-154), 24 NMR structures | ||||||
 Components Components | ACIDIC FIBROBLAST GROWTH FACTOR FGF1 FGF1 | ||||||
 Keywords Keywords |  GROWTH FACTOR / GROWTH FACTOR /  FIBROBLAST GROWTH FACTOR / ANTITUMORAL FIBROBLAST GROWTH FACTOR / ANTITUMORAL | ||||||
| Function / homology |  Function and homology information Function and homology informationmesonephric epithelium development / branch elongation involved in ureteric bud branching / regulation of endothelial tube morphogenesis / FGFR3b ligand binding and activation / regulation of endothelial cell chemotaxis to fibroblast growth factor / Signaling by activated point mutants of FGFR3 / FGFR3c ligand binding and activation / Phospholipase C-mediated cascade; FGFR3 / FGFR2b ligand binding and activation / positive regulation of cholesterol biosynthetic process ...mesonephric epithelium development / branch elongation involved in ureteric bud branching / regulation of endothelial tube morphogenesis / FGFR3b ligand binding and activation / regulation of endothelial cell chemotaxis to fibroblast growth factor / Signaling by activated point mutants of FGFR3 / FGFR3c ligand binding and activation / Phospholipase C-mediated cascade; FGFR3 / FGFR2b ligand binding and activation / positive regulation of cholesterol biosynthetic process /  fibroblast growth factor receptor binding / FGFR2c ligand binding and activation / Activated point mutants of FGFR2 / Phospholipase C-mediated cascade; FGFR2 / FGFR4 ligand binding and activation / FGFR1b ligand binding and activation / Phospholipase C-mediated cascade; FGFR4 / Signaling by activated point mutants of FGFR1 / FGFR1c ligand binding and activation / organ induction / Downstream signaling of activated FGFR1 / Phospholipase C-mediated cascade: FGFR1 / positive regulation of hepatocyte proliferation / S100 protein binding / positive regulation of intracellular signal transduction / Signaling by FGFR2 IIIa TM / PI-3K cascade:FGFR3 / positive regulation of sprouting angiogenesis / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / positive regulation of cell division / PI3K Cascade / anatomical structure morphogenesis / fibroblast growth factor receptor signaling pathway / SHC-mediated cascade:FGFR3 / SHC-mediated cascade:FGFR2 / SHC-mediated cascade:FGFR4 / SHC-mediated cascade:FGFR1 / FRS-mediated FGFR3 signaling / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / Signaling by FGFR3 in disease / FRS-mediated FGFR1 signaling / fibroblast growth factor receptor binding / FGFR2c ligand binding and activation / Activated point mutants of FGFR2 / Phospholipase C-mediated cascade; FGFR2 / FGFR4 ligand binding and activation / FGFR1b ligand binding and activation / Phospholipase C-mediated cascade; FGFR4 / Signaling by activated point mutants of FGFR1 / FGFR1c ligand binding and activation / organ induction / Downstream signaling of activated FGFR1 / Phospholipase C-mediated cascade: FGFR1 / positive regulation of hepatocyte proliferation / S100 protein binding / positive regulation of intracellular signal transduction / Signaling by FGFR2 IIIa TM / PI-3K cascade:FGFR3 / positive regulation of sprouting angiogenesis / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / positive regulation of cell division / PI3K Cascade / anatomical structure morphogenesis / fibroblast growth factor receptor signaling pathway / SHC-mediated cascade:FGFR3 / SHC-mediated cascade:FGFR2 / SHC-mediated cascade:FGFR4 / SHC-mediated cascade:FGFR1 / FRS-mediated FGFR3 signaling / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / Signaling by FGFR3 in disease / FRS-mediated FGFR1 signaling /  Hsp70 protein binding / Signaling by FGFR2 in disease / Signaling by FGFR1 in disease / activation of protein kinase B activity / Hsp70 protein binding / Signaling by FGFR2 in disease / Signaling by FGFR1 in disease / activation of protein kinase B activity /  extracellular matrix / positive regulation of endothelial cell migration / epithelial cell proliferation / positive regulation of epithelial cell proliferation / Negative regulation of FGFR3 signaling / Negative regulation of FGFR2 signaling / Negative regulation of FGFR4 signaling / Negative regulation of FGFR1 signaling / animal organ morphogenesis / lung development / extracellular matrix / positive regulation of endothelial cell migration / epithelial cell proliferation / positive regulation of epithelial cell proliferation / Negative regulation of FGFR3 signaling / Negative regulation of FGFR2 signaling / Negative regulation of FGFR4 signaling / Negative regulation of FGFR1 signaling / animal organ morphogenesis / lung development /  growth factor activity / positive regulation of MAP kinase activity / growth factor activity / positive regulation of MAP kinase activity /  wound healing / positive regulation of angiogenesis / Constitutive Signaling by Aberrant PI3K in Cancer / wound healing / positive regulation of angiogenesis / Constitutive Signaling by Aberrant PI3K in Cancer /  integrin binding / PIP3 activates AKT signaling / cellular response to heat / integrin binding / PIP3 activates AKT signaling / cellular response to heat /  heparin binding / heparin binding /  cell cortex / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade / cell cortex / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade /  angiogenesis / angiogenesis /  cell differentiation / positive regulation of ERK1 and ERK2 cascade / positive regulation of cell migration / positive regulation of cell population proliferation / positive regulation of gene expression / cell differentiation / positive regulation of ERK1 and ERK2 cascade / positive regulation of cell migration / positive regulation of cell population proliferation / positive regulation of gene expression /  signal transduction / positive regulation of transcription by RNA polymerase II / signal transduction / positive regulation of transcription by RNA polymerase II /  extracellular space / extracellular region / extracellular space / extracellular region /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  SOLUTION NMR / RESTRAINED MOLECULAR DYNAMICS SOLUTION NMR / RESTRAINED MOLECULAR DYNAMICS | ||||||
 Authors Authors | Lozano, R.M. / Pineda-Lucena, A. / Gonzalez, C. / Jimenez, M.A. / Cuevas, P. / Redondo-Horcajo, M. / Sanz, J.M. / Rico, M. / Gimenez-Gallego, G. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2000 Journal: Biochemistry / Year: 2000Title: 1H-NMR Structural Characterization of a Non Mitogenic, Vasodilatory, Ischemia-Protector and Neuromodulatory Acidic Fibroblast Growth Factor Authors: Lozano, R.M. / Pineda-Lucena, A. / Gonzalez, C. / Jimenez, M.A. / Cuevas, P. / Redonde-Horcajo, M. / Sanz, J.M. / Rico, M. / Gimenez-Gallego, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1dzd.cif.gz 1dzd.cif.gz | 483.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1dzd.ent.gz pdb1dzd.ent.gz | 397.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1dzd.json.gz 1dzd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dz/1dzd https://data.pdbj.org/pub/pdb/validation_reports/dz/1dzd ftp://data.pdbj.org/pub/pdb/validation_reports/dz/1dzd ftp://data.pdbj.org/pub/pdb/validation_reports/dz/1dzd | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  FGF1 FGF1Mass: 14357.054 Da / Num. of mol.: 1 / Fragment: RESIDUES 27-154 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HOMO SAPIENS (human) / Plasmid: PMG62 / Production host: HOMO SAPIENS (human) / Plasmid: PMG62 / Production host:   ESCHERICHIA COLI (E. coli) / References: UniProt: P05230 ESCHERICHIA COLI (E. coli) / References: UniProt: P05230 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Contents: WATER |
|---|---|
| Sample conditions | pH: 6.0 / Pressure: 1 atm / Temperature: 298 K |
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker AMX / Manufacturer: Bruker / Model : AMX / Field strength: 600 MHz : AMX / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: RESTRAINED MOLECULAR DYNAMICS / Software ordinal: 1 Details: REFINEMENT DETAILS CAN BE FOUND IN THE JRNL CITATION ABOVE | |||||||||
| NMR ensemble | Conformers calculated total number: 24 / Conformers submitted total number: 24 |
 Movie
Movie Controller
Controller













 PDBj
PDBj



