[English] 日本語
 Yorodumi
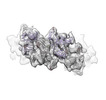
Yorodumi- EMDB-26424: Meprin alpha helix in complex with fetuin-B [consensus C1 reconst... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Meprin alpha helix in complex with fetuin-B [consensus C1 reconstruction] | |||||||||
 Map data Map data | B-factor amplitude corrected sharpened map, filtered by local resolution | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Bayly-Jones C / Lupton CJ / Fritz C / Schlenzig D / Whisstock JC | |||||||||
| Funding support |  Germany, Germany,  Australia, 2 items Australia, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Helical ultrastructure of the metalloprotease meprin α in complex with a small molecule inhibitor. Authors: Charles Bayly-Jones / Christopher J Lupton / Claudia Fritz / Hariprasad Venugopal / Daniel Ramsbeck / Michael Wermann / Christian Jäger / Alex de Marco / Stephan Schilling / Dagmar ...Authors: Charles Bayly-Jones / Christopher J Lupton / Claudia Fritz / Hariprasad Venugopal / Daniel Ramsbeck / Michael Wermann / Christian Jäger / Alex de Marco / Stephan Schilling / Dagmar Schlenzig / James C Whisstock /   Abstract: The zinc-dependent metalloprotease meprin α is predominantly expressed in the brush border membrane of proximal tubules in the kidney and enterocytes in the small intestine and colon. In normal ...The zinc-dependent metalloprotease meprin α is predominantly expressed in the brush border membrane of proximal tubules in the kidney and enterocytes in the small intestine and colon. In normal tissue homeostasis meprin α performs key roles in inflammation, immunity, and extracellular matrix remodelling. Dysregulated meprin α is associated with acute kidney injury, sepsis, urinary tract infection, metastatic colorectal carcinoma, and inflammatory bowel disease. Accordingly, meprin α is the target of drug discovery programs. In contrast to meprin β, meprin α is secreted into the extracellular space, whereupon it oligomerises to form giant assemblies and is the largest extracellular protease identified to date (~6 MDa). Here, using cryo-electron microscopy, we determine the high-resolution structure of the zymogen and mature form of meprin α, as well as the structure of the active form in complex with a prototype small molecule inhibitor and human fetuin-B. Our data reveal that meprin α forms a giant, flexible, left-handed helical assembly of roughly 22 nm in diameter. We find that oligomerisation improves proteolytic and thermal stability but does not impact substrate specificity or enzymatic activity. Furthermore, structural comparison with meprin β reveal unique features of the active site of meprin α, and helical assembly more broadly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26424.map.gz emd_26424.map.gz | 16.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26424-v30.xml emd-26424-v30.xml emd-26424.xml emd-26424.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26424_fsc.xml emd_26424_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_26424.png emd_26424.png | 108.3 KB | ||
| Masks |  emd_26424_msk_1.map emd_26424_msk_1.map | 103 MB |  Mask map Mask map | |
| Others |  emd_26424_additional_1.map.gz emd_26424_additional_1.map.gz emd_26424_half_map_1.map.gz emd_26424_half_map_1.map.gz emd_26424_half_map_2.map.gz emd_26424_half_map_2.map.gz | 51.8 MB 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26424 http://ftp.pdbj.org/pub/emdb/structures/EMD-26424 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26424 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26424 | HTTPS FTP |
-Validation report
| Summary document |  emd_26424_validation.pdf.gz emd_26424_validation.pdf.gz | 918 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26424_full_validation.pdf.gz emd_26424_full_validation.pdf.gz | 917.6 KB | Display | |
| Data in XML |  emd_26424_validation.xml.gz emd_26424_validation.xml.gz | 18.4 KB | Display | |
| Data in CIF |  emd_26424_validation.cif.gz emd_26424_validation.cif.gz | 23.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26424 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26424 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26424 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26424 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26424.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26424.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor amplitude corrected sharpened map, filtered by local resolution | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4133 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26424_msk_1.map emd_26424_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened final map. Low pass filtered to the global FSC.
| File | emd_26424_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened final map. Low pass filtered to the global FSC. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half-map (1 of 2).
| File | emd_26424_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half-map (1 of 2). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half-map (2 of 2).
| File | emd_26424_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half-map (2 of 2). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Inhibitory complex of meprin alpha helix with fetuin-B [consensus...
| Entire | Name: Inhibitory complex of meprin alpha helix with fetuin-B [consensus C1 reconstruction] |
|---|---|
| Components |
|
-Supramolecule #1: Inhibitory complex of meprin alpha helix with fetuin-B [consensus...
| Supramolecule | Name: Inhibitory complex of meprin alpha helix with fetuin-B [consensus C1 reconstruction] type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all Details: Consensus standard reconstruction of full helix of recombinant, secreted helical pro-meprin alpha |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 85 kDa/nm |
-Macromolecule #1: Meprin alpha
| Macromolecule | Name: Meprin alpha / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: WSHPQFEKVP IKYLPEENVH DADFGEQKDI SEINLAAGLD LFQGDIL LQ KSRNGLRDPN TRWTFPIPYI LADNLGLNAK GAILYAFEMF RLKSCVDFKP YEGESSYI I FQQFDGCWSE VGDQHVGQNI SIGQGCAYKA IIEHEILHAL GFYHEQSRTD RDDYVNIWW ...String: WSHPQFEKVP IKYLPEENVH DADFGEQKDI SEINLAAGLD LFQGDIL LQ KSRNGLRDPN TRWTFPIPYI LADNLGLNAK GAILYAFEMF RLKSCVDFKP YEGESSYI I FQQFDGCWSE VGDQHVGQNI SIGQGCAYKA IIEHEILHAL GFYHEQSRTD RDDYVNIWW DQILSGYQHN FDTYDDSLIT DLNTPYDYES LMHYQPFSFN KNASVPTITA KIPEFNSIIG QRLDFSAID LERLNRMYNC TTTHTLLDHC TFEKANICGM IQGTRDDTDW AHQDSAQAGE V DHTLLGQC TGAGYFMQFS TSSGSAEEAA LLESRILYPK RKQQCLQFFY KMTGSPSDRL VV WVRRDDS TGNVRKLVKV QTFQGDDDHN WKIAHVVLKE EQKFRYLFQG TKGDPQNSTG GIY LDDITL TETPCPTGVW TVRNFSQVLE NTSKGDKLQS PRFYNSEGYG FGVTLYPNSR ESSG YLRLA FHVCSGENDA ILEWPVENRQ VIITILDQEP DVRNRMSSSM VFTTSKSHTS PAIND TVIW DRPSRVGTYH TDCNCFRSID LGWSGFISHQ MLKRRSFLKN DDLIIFVDFE DITHLS |
-Macromolecule #2: fetuin-B
| Macromolecule | Name: fetuin-B / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGLLLPLALC ILVLCCGAMS PPQLALNPSA LLSRGCNDSD VLAVAGFALR DINKDRKDGY VLRLNRVND AQEYRRGGLG SLFYLTLDVL ETDCHVLRKK AWQDCGMRIF FESVYGQCKA I FYMNNPSR VLYLAAYNCT LRPVSKKKIY MTCPDCPSSI PTDSSNHQVL ...String: MGLLLPLALC ILVLCCGAMS PPQLALNPSA LLSRGCNDSD VLAVAGFALR DINKDRKDGY VLRLNRVND AQEYRRGGLG SLFYLTLDVL ETDCHVLRKK AWQDCGMRIF FESVYGQCKA I FYMNNPSR VLYLAAYNCT LRPVSKKKIY MTCPDCPSSI PTDSSNHQVL EAATESLAKY NN ENTSKQY SLFKVTRASS QWVVGPSYFV EYLIKESPCT KSQASSCSLQ SSDSVPVGLC KGS LTRTHW EKFVSVTCDF FESQAPATGS ENSAVNQKPT NLPKVEESQQ KNTPPTDSPS KAGP RGSVQ YLPDLDDKNS QEKGPQEAFP VHLDLTTNPQ GETLDISFLF LEPMEEKLVV LPFPK EKAR TAECPGPAQN ASPLVLPPHH HHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: 3 s blot, -3 force. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-25 / Number grids imaged: 1 / Number real images: 4068 / Average electron dose: 44.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X



































