+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tube assembly of Atg18-PR72AA | |||||||||
 Map data Map data | sharpened map from CryoSPARC software | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  autophagy / membrane remodeling / PIP binding / autophagy / membrane remodeling / PIP binding /  PI3P / PI(3 / 5)P2 / lipid binding protein / PI3P / PI(3 / 5)P2 / lipid binding protein /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of phosphatidylinositol biosynthetic process / PAS complex / 1-phosphatidyl-1D-myo-inositol 3,5-bisphosphate metabolic process / phagophore /  vacuolar protein processing / positive regulation of vacuole organization / vacuolar protein processing / positive regulation of vacuole organization /  Macroautophagy / cytoplasm to vacuole targeting by the Cvt pathway / nucleophagy / pexophagy ...regulation of phosphatidylinositol biosynthetic process / PAS complex / 1-phosphatidyl-1D-myo-inositol 3,5-bisphosphate metabolic process / phagophore / Macroautophagy / cytoplasm to vacuole targeting by the Cvt pathway / nucleophagy / pexophagy ...regulation of phosphatidylinositol biosynthetic process / PAS complex / 1-phosphatidyl-1D-myo-inositol 3,5-bisphosphate metabolic process / phagophore /  vacuolar protein processing / positive regulation of vacuole organization / vacuolar protein processing / positive regulation of vacuole organization /  Macroautophagy / cytoplasm to vacuole targeting by the Cvt pathway / nucleophagy / pexophagy / protein localization to phagophore assembly site / piecemeal microautophagy of the nucleus / phagophore assembly site membrane / late endosome to vacuole transport / Macroautophagy / cytoplasm to vacuole targeting by the Cvt pathway / nucleophagy / pexophagy / protein localization to phagophore assembly site / piecemeal microautophagy of the nucleus / phagophore assembly site membrane / late endosome to vacuole transport /  phosphatidylinositol-3-phosphate binding / fungal-type vacuole membrane / phagophore assembly site / phosphatidylinositol-4-phosphate binding / phosphatidylinositol-3,5-bisphosphate binding / vacuolar membrane / phosphatidylinositol-3-phosphate binding / fungal-type vacuole membrane / phagophore assembly site / phosphatidylinositol-4-phosphate binding / phosphatidylinositol-3,5-bisphosphate binding / vacuolar membrane /  extrinsic component of membrane / extrinsic component of membrane /  autophagosome assembly / autophagosome assembly /  ubiquitin binding / cell periphery / ubiquitin binding / cell periphery /  macroautophagy / macroautophagy /  protein transport / endosome membrane / protein transport / endosome membrane /  endosome / protein-containing complex / endosome / protein-containing complex /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | |||||||||
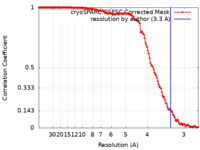
| Method | helical reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Mann D / Fromm S / Martinez-Sanchez A / Gopaldass N / Mayer A / Sachse C | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structures of Atg18 oligomers reveal a tilted structural scaffold for Atg2 at the isolation membrane Authors: Mann D / Fromm SA / Martinez-Sanchez A / Gopaldass N / Mayer A / Sachse C | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15408.map.gz emd_15408.map.gz | 229.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15408-v30.xml emd-15408-v30.xml emd-15408.xml emd-15408.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15408_fsc.xml emd_15408_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_15408.png emd_15408.png | 284.1 KB | ||
| Masks |  emd_15408_msk_1.map emd_15408_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15408.cif.gz emd-15408.cif.gz | 5.7 KB | ||
| Others |  emd_15408_additional_1.map.gz emd_15408_additional_1.map.gz emd_15408_half_map_1.map.gz emd_15408_half_map_1.map.gz emd_15408_half_map_2.map.gz emd_15408_half_map_2.map.gz | 186.4 MB 226.2 MB 226.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15408 http://ftp.pdbj.org/pub/emdb/structures/EMD-15408 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15408 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15408 | HTTPS FTP |
-Related structure data
| Related structure data |  8afqMC  8afwC  8afxC  8afyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15408.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15408.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map from CryoSPARC software | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||
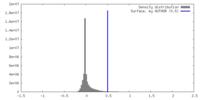
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15408_msk_1.map emd_15408_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
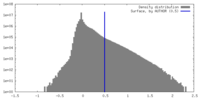
| Density Histograms |
-Additional map: autosharpened map from Phenix software
| File | emd_15408_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | autosharpened map from Phenix software | ||||||||||||
| Projections & Slices |
| ||||||||||||
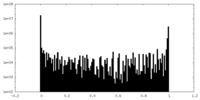
| Density Histograms |
-Half map: half map A
| File | emd_15408_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
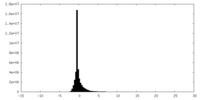
| Density Histograms |
-Half map: half map B
| File | emd_15408_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Filament assembly of Atg18-PR72AA
| Entire | Name: Filament assembly of Atg18-PR72AA |
|---|---|
| Components |
|
-Supramolecule #1: Filament assembly of Atg18-PR72AA
| Supramolecule | Name: Filament assembly of Atg18-PR72AA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
-Macromolecule #1: Autophagy-related protein 18
| Macromolecule | Name: Autophagy-related protein 18 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) / Strain: ATCC 204508 / S288c Saccharomyces cerevisiae (brewer's yeast) / Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 55.045777 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MSDSSPTINF INFNQTGTCI SLGTSKGFKI FNCEPFGKFY SEDSGGYAIV EMLFSTSLLA LVGIGDQPAL SAARLRIINT KKHSIICEV TFPTSILSVK MNKSRLVVLL QEQIYIYDIN TMRLLHTIET NPNPRGLMAM SPSVANSYLV YPSPPKVINS E IKAHATTN ...String: MSDSSPTINF INFNQTGTCI SLGTSKGFKI FNCEPFGKFY SEDSGGYAIV EMLFSTSLLA LVGIGDQPAL SAARLRIINT KKHSIICEV TFPTSILSVK MNKSRLVVLL QEQIYIYDIN TMRLLHTIET NPNPRGLMAM SPSVANSYLV YPSPPKVINS E IKAHATTN NITLSVGGNT ETSFKRDQQD AGHSDISDLD QYSSFTKRDD ADPTSSNGGN SSIIKNGDVI VFNLETLQPT MV IEAHKGE IAAMAISFDG TLMATASDKG TIIRVFDIET GDKIYQFRRG TYATRIYSIS FSEDSQYLAV TGSSKTVHIF KLG HSMSNN KLDSDDSNME EAAADDSSLD TTSIDALSDE ENPTRLAREP YVDASRKTMG RMIRYSSQKL SRRAARTLGQ IFPI KVTSL LESSRHFASL KLPVETNSHV MTISSIGSPI DIDTSEYPEL FETGNSASTE SYHEPVMKMV PIRVVSSDGY LYNFV MDPE RGGDCLILSQ YSILMD UniProtKB: Autophagy-related protein 18 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Grid | Model: UltrAuFoil R1.2/1.3 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 99 % / Chamber temperature: 291 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 2380 / Average electron dose: 90.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8afq: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)