[English] 日本語
 Yorodumi
Yorodumi- EMDB-30002: Cryo-EM structure of TLR7/Cpd-7 (DSR-139970) complex in open form -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30002 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of TLR7/Cpd-7 (DSR-139970) complex in open form | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationtoll-like receptor 7 signaling pathway / response to cGMP / siRNA binding / early phagosome / positive regulation of macrophage cytokine production /  pattern recognition receptor activity / pattern recognition receptor activity /  toll-like receptor signaling pathway / positive regulation of interferon-alpha production / canonical NF-kappaB signal transduction / positive regulation of chemokine production ...toll-like receptor 7 signaling pathway / response to cGMP / siRNA binding / early phagosome / positive regulation of macrophage cytokine production / toll-like receptor signaling pathway / positive regulation of interferon-alpha production / canonical NF-kappaB signal transduction / positive regulation of chemokine production ...toll-like receptor 7 signaling pathway / response to cGMP / siRNA binding / early phagosome / positive regulation of macrophage cytokine production /  pattern recognition receptor activity / pattern recognition receptor activity /  toll-like receptor signaling pathway / positive regulation of interferon-alpha production / canonical NF-kappaB signal transduction / positive regulation of chemokine production / JNK cascade / positive regulation of interferon-beta production / positive regulation of interleukin-8 production / toll-like receptor signaling pathway / positive regulation of interferon-alpha production / canonical NF-kappaB signal transduction / positive regulation of chemokine production / JNK cascade / positive regulation of interferon-beta production / positive regulation of interleukin-8 production /  regulation of protein phosphorylation / cellular response to virus / positive regulation of interleukin-6 production / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to mechanical stimulus / positive regulation of type II interferon production / regulation of protein phosphorylation / cellular response to virus / positive regulation of interleukin-6 production / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to mechanical stimulus / positive regulation of type II interferon production /  double-stranded RNA binding / defense response to virus / double-stranded RNA binding / defense response to virus /  lysosome / lysosome /  single-stranded RNA binding / single-stranded RNA binding /  receptor complex / receptor complex /  endosome / endosome /  inflammatory response / inflammatory response /  innate immune response / innate immune response /  endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Macaca mulatta (Rhesus monkey) Macaca mulatta (Rhesus monkey) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.8 Å cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Zhang Z / Ohto U / Shimizu T | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structural analysis reveals TLR7 dynamics underlying antagonism. Authors: Shingo Tojo / Zhikuan Zhang / Hiroyuki Matsui / Masahiro Tahara / Mitsunori Ikeguchi / Mami Kochi / Mami Kamada / Hideki Shigematsu / Akihisa Tsutsumi / Naruhiko Adachi / Takuma Shibata / ...Authors: Shingo Tojo / Zhikuan Zhang / Hiroyuki Matsui / Masahiro Tahara / Mitsunori Ikeguchi / Mami Kochi / Mami Kamada / Hideki Shigematsu / Akihisa Tsutsumi / Naruhiko Adachi / Takuma Shibata / Masaki Yamamoto / Masahide Kikkawa / Toshiya Senda / Yoshiaki Isobe / Umeharu Ohto / Toshiyuki Shimizu /  Abstract: Toll-like receptor 7 (TLR7) recognizes both microbial and endogenous RNAs and nucleosides. Aberrant activation of TLR7 has been implicated in several autoimmune diseases including systemic lupus ...Toll-like receptor 7 (TLR7) recognizes both microbial and endogenous RNAs and nucleosides. Aberrant activation of TLR7 has been implicated in several autoimmune diseases including systemic lupus erythematosus (SLE). Here, by modifying potent TLR7 agonists, we develop a series of TLR7-specific antagonists as promising therapeutic agents for SLE. These compounds protect mice against lethal autoimmunity. Combining crystallography and cryo-electron microscopy, we identify the open conformation of the receptor and reveal the structural equilibrium between open and closed conformations that underlies TLR7 antagonism, as well as the detailed mechanism by which TLR7-specific antagonists bind to their binding pocket in TLR7. Our work provides small-molecule TLR7-specific antagonists and suggests the TLR7-targeting strategy for treating autoimmune diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30002.map.gz emd_30002.map.gz | 38 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30002-v30.xml emd-30002-v30.xml emd-30002.xml emd-30002.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30002.png emd_30002.png | 240 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30002 http://ftp.pdbj.org/pub/emdb/structures/EMD-30002 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30002 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30002 | HTTPS FTP |
-Related structure data
| Related structure data |  6lw1MC  0999C  1000C  6lvxC  6lvyC  6lvzC  6lw0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30002.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30002.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.996 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : TLR7/Cpd-7 (DSR-139970) complex
| Entire | Name: TLR7/Cpd-7 (DSR-139970) complex |
|---|---|
| Components |
|
-Supramolecule #1: TLR7/Cpd-7 (DSR-139970) complex
| Supramolecule | Name: TLR7/Cpd-7 (DSR-139970) complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Macaca mulatta (Rhesus monkey) Macaca mulatta (Rhesus monkey) |
| Recombinant expression | Organism:   Drosophila melanogaster (fruit fly) Drosophila melanogaster (fruit fly) |
| Molecular weight | Theoretical: 200 KDa |
-Macromolecule #1: Toll-like receptor 7
| Macromolecule | Name: Toll-like receptor 7 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Macaca mulatta (Rhesus monkey) Macaca mulatta (Rhesus monkey) |
| Molecular weight | Theoretical: 94.745594 KDa |
| Recombinant expression | Organism:   Drosophila melanogaster (fruit fly) Drosophila melanogaster (fruit fly) |
| Sequence | String: RSPWARWFPK TLPCDVTLDV SKNHVIVDCT DKHLTEIPGG IPTNTTNLTL TINHIPDISP ASFHRLVHLV EIDFRCNCVP IRLGSKSNM CPRRLQIKPR SFSGLTYLKS LYLDGNQLLE IPQGLPPSLQ LLSLEANNIF SIRKEQLTEL ANIEILYLGQ N CYYRNPCY ...String: RSPWARWFPK TLPCDVTLDV SKNHVIVDCT DKHLTEIPGG IPTNTTNLTL TINHIPDISP ASFHRLVHLV EIDFRCNCVP IRLGSKSNM CPRRLQIKPR SFSGLTYLKS LYLDGNQLLE IPQGLPPSLQ LLSLEANNIF SIRKEQLTEL ANIEILYLGQ N CYYRNPCY VSYSIEKDAF LNLTKLKVLS LKDNNVTTVP TVLPSTLTEL YLYNNMIAEI QEDDFNNLNQ LQILDLSGNC PR CYNAPFP CTPCKNNSPL QIPVNAFDAL TELKVLRLHS NSLQHVPPRW FKNINNLQEL DLSQNFLAKE IGDAKFLHFL PNL IQLDLS FNFELQVYRA SMNLSQAFSS LKSLKILRIR GYVFKELKSF QLSPLHNLQN LEVLDLGTNF IKIANLSMFK QFKR LKVID LSVNKISPSG DSLVPRGSSN ARTSVESYEP QVLEQLYYFR YDKYARSCRF KNKEASFTSV QESCYKYGQT LDLSK NSIF FIKSSDFQHL SFLKCLNLSG NLISQTLNGS EFQPLAELRY LDFSNNRLDL LHSTAFEELR KLEVLDISSN SHYFQS EGI THMLNFTKNL KVLQKLMMND NDISSSTSRT MESESLRTLE FRGNHLDVLW RDGDNRYLQL FKNLLKLEEL DISKNSL SF LPSGVFDGMP PNLKNLSLAK NGLKSFIWEK LRYLKNLETL DLSHNQLTTV PERLSNCSRS LKNLILKNNQ IRSLTKYF L QDAFQLRYLD LSSNKIQMIQ KTSFPENVLN NLKMLLLHHN RFLCTCDAVW FVWWVQHTEV TIPYLATDVT CVGPGAHKG QSVISLDLYT CELDLTNEFL VPR |
-Macromolecule #2: 2-ethoxy-8-(5-fluoranylpyridin-3-yl)-6-methyl-9-[[4-[[(1S,4S)-5-m...
| Macromolecule | Name: 2-ethoxy-8-(5-fluoranylpyridin-3-yl)-6-methyl-9-[[4-[[(1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl]methyl]phenyl]methyl]purine type: ligand / ID: 2 / Number of copies: 2 / Formula: EX3 |
|---|---|
| Molecular weight | Theoretical: 487.572 Da |
| Chemical component information | 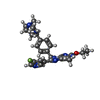 ChemComp-EX3: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 4.8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Initial angle assignment | Type: NOT APPLICABLE |
|---|---|
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 301212 ) / Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 301212 |
 Movie
Movie Controller
Controller
















