[English] 日本語
 Yorodumi
Yorodumi- PDB-6hay: Crystal structure of PROTAC 1 in complex with the bromodomain of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6hay | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of PROTAC 1 in complex with the bromodomain of human SMARCA2 and pVHL:ElonginC:ElonginB | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  GENE REGULATION / GENE REGULATION /  bromodomain / E3 Ubiquitin Protein Ligase bromodomain / E3 Ubiquitin Protein Ligase | ||||||
| Function / homology |  Function and homology information Function and homology informationbBAF complex / npBAF complex /  brahma complex / brahma complex /  nBAF complex / regulation of cellular response to hypoxia / GBAF complex / regulation of G0 to G1 transition / RHOBTB3 ATPase cycle / negative regulation of receptor signaling pathway via JAK-STAT / transcription elongation factor activity ...bBAF complex / npBAF complex / nBAF complex / regulation of cellular response to hypoxia / GBAF complex / regulation of G0 to G1 transition / RHOBTB3 ATPase cycle / negative regulation of receptor signaling pathway via JAK-STAT / transcription elongation factor activity ...bBAF complex / npBAF complex /  brahma complex / brahma complex /  nBAF complex / regulation of cellular response to hypoxia / GBAF complex / regulation of G0 to G1 transition / RHOBTB3 ATPase cycle / negative regulation of receptor signaling pathway via JAK-STAT / transcription elongation factor activity / regulation of nucleotide-excision repair / target-directed miRNA degradation / elongin complex / VCB complex / nBAF complex / regulation of cellular response to hypoxia / GBAF complex / regulation of G0 to G1 transition / RHOBTB3 ATPase cycle / negative regulation of receptor signaling pathway via JAK-STAT / transcription elongation factor activity / regulation of nucleotide-excision repair / target-directed miRNA degradation / elongin complex / VCB complex /  SWI/SNF complex / ATP-dependent chromatin remodeler activity / regulation of mitotic metaphase/anaphase transition / Replication of the SARS-CoV-1 genome / Cul5-RING ubiquitin ligase complex / positive regulation of double-strand break repair / positive regulation of T cell differentiation / intermediate filament cytoskeleton / Cul2-RING ubiquitin ligase complex / positive regulation of stem cell population maintenance / intracellular non-membrane-bounded organelle / SUMOylation of ubiquitinylation proteins / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / negative regulation of transcription elongation by RNA polymerase II / spermatid development / regulation of G1/S transition of mitotic cell cycle / negative regulation of cell differentiation / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / ubiquitin-like ligase-substrate adaptor activity / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / positive regulation of myoblast differentiation / ATP-dependent activity, acting on DNA / Formation of HIV elongation complex in the absence of HIV Tat / negative regulation of signal transduction / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / negative regulation of TORC1 signaling / RNA Polymerase II Pre-transcription Events / negative regulation of autophagy / transcription corepressor binding / SWI/SNF complex / ATP-dependent chromatin remodeler activity / regulation of mitotic metaphase/anaphase transition / Replication of the SARS-CoV-1 genome / Cul5-RING ubiquitin ligase complex / positive regulation of double-strand break repair / positive regulation of T cell differentiation / intermediate filament cytoskeleton / Cul2-RING ubiquitin ligase complex / positive regulation of stem cell population maintenance / intracellular non-membrane-bounded organelle / SUMOylation of ubiquitinylation proteins / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / negative regulation of transcription elongation by RNA polymerase II / spermatid development / regulation of G1/S transition of mitotic cell cycle / negative regulation of cell differentiation / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / ubiquitin-like ligase-substrate adaptor activity / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / positive regulation of myoblast differentiation / ATP-dependent activity, acting on DNA / Formation of HIV elongation complex in the absence of HIV Tat / negative regulation of signal transduction / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / negative regulation of TORC1 signaling / RNA Polymerase II Pre-transcription Events / negative regulation of autophagy / transcription corepressor binding /  helicase activity / transcription elongation by RNA polymerase II / helicase activity / transcription elongation by RNA polymerase II /  transcription initiation at RNA polymerase II promoter / positive regulation of cell differentiation / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / positive regulation of cell differentiation / TP53 Regulates Transcription of DNA Repair Genes /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Vif-mediated degradation of APOBEC3G / cell morphogenesis / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Inactivation of CSF3 (G-CSF) signaling / negative regulation of cell growth / RMTs methylate histone arginines / Regulation of expression of SLITs and ROBOs / ubiquitin-protein transferase activity / transcription corepressor activity / protein-macromolecule adaptor activity / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / Antigen processing: Ubiquitination & Proteasome degradation / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Vif-mediated degradation of APOBEC3G / cell morphogenesis / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Inactivation of CSF3 (G-CSF) signaling / negative regulation of cell growth / RMTs methylate histone arginines / Regulation of expression of SLITs and ROBOs / ubiquitin-protein transferase activity / transcription corepressor activity / protein-macromolecule adaptor activity / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / Antigen processing: Ubiquitination & Proteasome degradation /  Neddylation / Neddylation /  nervous system development / Replication of the SARS-CoV-2 genome / nervous system development / Replication of the SARS-CoV-2 genome /  histone binding / ubiquitin-dependent protein catabolic process / cellular response to hypoxia / proteasome-mediated ubiquitin-dependent protein catabolic process / histone binding / ubiquitin-dependent protein catabolic process / cellular response to hypoxia / proteasome-mediated ubiquitin-dependent protein catabolic process /  regulation of gene expression / protein-containing complex assembly / DNA-binding transcription factor binding / amyloid fibril formation / regulation of gene expression / protein-containing complex assembly / DNA-binding transcription factor binding / amyloid fibril formation /  transcription coactivator activity / molecular adaptor activity / protein stabilization / protein ubiquitination / transcription cis-regulatory region binding / transcription coactivator activity / molecular adaptor activity / protein stabilization / protein ubiquitination / transcription cis-regulatory region binding /  hydrolase activity / hydrolase activity /  chromatin remodeling / negative regulation of cell population proliferation / negative regulation of gene expression / intracellular membrane-bounded organelle / negative regulation of DNA-templated transcription / chromatin remodeling / negative regulation of cell population proliferation / negative regulation of gene expression / intracellular membrane-bounded organelle / negative regulation of DNA-templated transcription /  ubiquitin protein ligase binding / ubiquitin protein ligase binding /  chromatin binding / positive regulation of cell population proliferation / chromatin binding / positive regulation of cell population proliferation /  chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II /  enzyme binding / enzyme binding /  endoplasmic reticulum / positive regulation of transcription by RNA polymerase II endoplasmic reticulum / positive regulation of transcription by RNA polymerase IISimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.24 Å molecular replacement / Resolution: 2.24 Å | ||||||
 Authors Authors | Roy, M. / Bader, G. / Diers, E. / Trainor, N. / Farnaby, W. / Ciulli, A. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat.Chem.Biol. / Year: 2019 Journal: Nat.Chem.Biol. / Year: 2019Title: BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Authors: Farnaby, W. / Koegl, M. / Roy, M.J. / Whitworth, C. / Diers, E. / Trainor, N. / Zollman, D. / Steurer, S. / Karolyi-Oezguer, J. / Riedmueller, C. / Gmaschitz, T. / Wachter, J. / Dank, C. / ...Authors: Farnaby, W. / Koegl, M. / Roy, M.J. / Whitworth, C. / Diers, E. / Trainor, N. / Zollman, D. / Steurer, S. / Karolyi-Oezguer, J. / Riedmueller, C. / Gmaschitz, T. / Wachter, J. / Dank, C. / Galant, M. / Sharps, B. / Rumpel, K. / Traxler, E. / Gerstberger, T. / Schnitzer, R. / Petermann, O. / Greb, P. / Weinstabl, H. / Bader, G. / Zoephel, A. / Weiss-Puxbaum, A. / Ehrenhofer-Wolfer, K. / Wohrle, S. / Boehmelt, G. / Rinnenthal, J. / Arnhof, H. / Wiechens, N. / Wu, M.Y. / Owen-Hughes, T. / Ettmayer, P. / Pearson, M. / McConnell, D.B. / Ciulli, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6hay.cif.gz 6hay.cif.gz | 407.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6hay.ent.gz pdb6hay.ent.gz | 331.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6hay.json.gz 6hay.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ha/6hay https://data.pdbj.org/pub/pdb/validation_reports/ha/6hay ftp://data.pdbj.org/pub/pdb/validation_reports/ha/6hay ftp://data.pdbj.org/pub/pdb/validation_reports/ha/6hay | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6haxC  6hazC  6hr2C 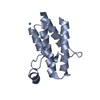 4qy4S  5t35S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 4 types, 8 molecules AEBFCGDH
| #1: Protein | Mass: 14380.542 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SMARCA2, BAF190B, BRM, SNF2A, SNF2L2 / Plasmid: pDEST15 / Production host: Homo sapiens (human) / Gene: SMARCA2, BAF190B, BRM, SNF2A, SNF2L2 / Plasmid: pDEST15 / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) Escherichia coli (E. coli) / Strain (production host): BL21(DE3)References: UniProt: P51531,  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement#2: Protein | Mass: 18702.291 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: VHL / Plasmid: pHAT4 / Production host: Homo sapiens (human) / Gene: VHL / Plasmid: pHAT4 / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P40337 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P40337#3: Protein | Mass: 10974.616 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: ELOC, TCEB1 / Plasmid: pCDF-1b DUET / Production host: Homo sapiens (human) / Gene: ELOC, TCEB1 / Plasmid: pCDF-1b DUET / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q15369 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q15369#4: Protein | Mass: 11748.406 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: ELOB, TCEB2 / Plasmid: pCDF-1b DUET / Production host: Homo sapiens (human) / Gene: ELOB, TCEB2 / Plasmid: pCDF-1b DUET / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q15370 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q15370 |
|---|
-Non-polymers , 5 types, 326 molecules 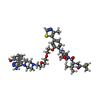








| #5: Chemical | | #6: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#7: Chemical | ChemComp-FMT / |  Formic acid Formic acid#8: Chemical | ChemComp-EPE / |  HEPES HEPES#9: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.79 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.75 Details: 18% (w/v) PEG 3350, 0.2 M sodium formate, 0.1 M HEPES, pH 7.75 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.91587 Å / Beamline: I04-1 / Wavelength: 0.91587 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Jul 30, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.91587 Å / Relative weight: 1 : 0.91587 Å / Relative weight: 1 |
| Reflection | Resolution: 2.24→48.33 Å / Num. obs: 105978 / % possible obs: 99.6 % / Redundancy: 6.97 % / Biso Wilson estimate: 52.59 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.081 / Rrim(I) all: 0.088 / Net I/σ(I): 16.1 |
| Reflection shell | Resolution: 2.24→2.37 Å / Redundancy: 6.94 % / Rmerge(I) obs: 0.851 / Mean I/σ(I) obs: 2 / Num. unique obs: 16832 / CC1/2: 0.81 / Rrim(I) all: 0.919 / % possible all: 97.9 |
-Phasing
Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5T35, 4QY4 Resolution: 2.24→48.33 Å / Cor.coef. Fo:Fc: 0.947 / Cor.coef. Fo:Fc free: 0.938 / Rfactor Rfree error: 0 / SU R Cruickshank DPI: 0.252 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.254 / SU Rfree Blow DPI: 0.188 / SU Rfree Cruickshank DPI: 0.19
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 165.73 Å2 / Biso mean: 55.66 Å2 / Biso min: 25.09 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.28 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.24→48.33 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.24→2.3 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


















