+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4zkb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The chemokine binding protein of orf virus complexed with CCL3 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | Viral Protein/cytokine / Complex / Orf chemokine binding protein / CCL3 / Viral Protein-cytokine complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationlymphocyte chemotaxis / granulocyte chemotaxis / CCR1 chemokine receptor binding / positive regulation of natural killer cell chemotaxis / positive regulation of microglial cell migration / astrocyte cell migration / regulation of behavior / CCR5 chemokine receptor binding / eosinophil degranulation / regulation of sensory perception of pain ...lymphocyte chemotaxis / granulocyte chemotaxis / CCR1 chemokine receptor binding / positive regulation of natural killer cell chemotaxis / positive regulation of microglial cell migration / astrocyte cell migration / regulation of behavior / CCR5 chemokine receptor binding / eosinophil degranulation / regulation of sensory perception of pain / CCR chemokine receptor binding / signaling / negative regulation of bone mineralization / positive regulation of microglial cell activation / cell activation / T cell chemotaxis / eosinophil chemotaxis / response to cholesterol / positive regulation of calcium ion transport / chemokine activity / Chemokine receptors bind chemokines / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / chemokine-mediated signaling pathway / phospholipase activator activity / positive regulation of calcium ion import / chemoattractant activity / negative regulation of osteoclast differentiation / Interleukin-10 signaling / exocytosis / macrophage chemotaxis / monocyte chemotaxis / host-mediated suppression of viral transcription / cellular response to interleukin-1 / neutrophil chemotaxis / cytoskeleton organization / positive regulation of calcium-mediated signaling / positive regulation of interleukin-1 beta production / cell chemotaxis / calcium-mediated signaling / cellular response to type II interferon / response to toxic substance / chemotaxis / intracellular calcium ion homeostasis / positive regulation of tumor necrosis factor production / positive regulation of inflammatory response / osteoblast differentiation / kinase activity / cellular response to tumor necrosis factor / calcium ion transport / cell-cell signaling / antimicrobial humoral immune response mediated by antimicrobial peptide / positive regulation of neuron apoptotic process / regulation of cell shape / MAPK cascade / protein kinase activity / positive regulation of ERK1 and ERK2 cascade / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of cell migration / inflammatory response / negative regulation of gene expression / positive regulation of gene expression / extracellular space / extracellular region / identical protein binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Orf virus Orf virus Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.9 Å MOLECULAR REPLACEMENT / Resolution: 2.9 Å | |||||||||
 Authors Authors | Knapp, K.M. / Nakatani, Y. / Krause, K.L. | |||||||||
 Citation Citation |  Journal: Structure / Year: 2015 Journal: Structure / Year: 2015Title: Structures of Orf Virus Chemokine Binding Protein in Complex with Host Chemokines Reveal Clues to Broad Binding Specificity. Authors: Counago, R.M. / Knapp, K.M. / Nakatani, Y. / Fleming, S.B. / Corbett, M. / Wise, L.M. / Mercer, A.A. / Krause, K.L. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4zkb.cif.gz 4zkb.cif.gz | 116.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4zkb.ent.gz pdb4zkb.ent.gz | 87.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4zkb.json.gz 4zkb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zk/4zkb https://data.pdbj.org/pub/pdb/validation_reports/zk/4zkb ftp://data.pdbj.org/pub/pdb/validation_reports/zk/4zkb ftp://data.pdbj.org/pub/pdb/validation_reports/zk/4zkb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4p5iSC  4zk9C  4zkcC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 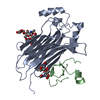
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 30374.613 Da / Num. of mol.: 1 / Fragment: UNP residues 17-286 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Orf virus (strain NZ2) / Plasmid: PTT5 / Cell line (production host): HEK 293-6E / Production host: Orf virus (strain NZ2) / Plasmid: PTT5 / Cell line (production host): HEK 293-6E / Production host:  Homo sapiens (human) / References: UniProt: Q2F862 Homo sapiens (human) / References: UniProt: Q2F862 |
|---|---|
| #2: Protein | Mass: 8551.468 Da / Num. of mol.: 1 / Fragment: UNP residues 24-92 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CCL3, G0S19-1, MIP1A, SCYA3 / Plasmid: pTT5 / Cell line (production host): HEK 293-6E / Production host: Homo sapiens (human) / Gene: CCL3, G0S19-1, MIP1A, SCYA3 / Plasmid: pTT5 / Cell line (production host): HEK 293-6E / Production host:  Homo sapiens (human) / References: UniProt: P10147 Homo sapiens (human) / References: UniProt: P10147 |
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
| #4: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.63 Å3/Da / Density % sol: 66.16 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 5.6 Details: 0.2M potassium sodium tartrate tetrahydrate, 0.1M sodium citrate tribasic dihydrate pH5.6, 2.0M ammonium citrate |
-Data collection
| Diffraction | Mean temperature: 93.2 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX1 / Wavelength: 0.95369 Å / Beamline: MX1 / Wavelength: 0.95369 Å |
| Detector | Type: ADSC QUANTUM 210r / Detector: CCD / Date: Mar 10, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.95369 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→47.44 Å / Num. obs: 13416 / % possible obs: 99.8 % / Redundancy: 4.9 % / Rmerge(I) obs: 0.117 / Net I/σ(I): 8.4 |
| Reflection shell | Resolution: 2.9→3.06 Å / Redundancy: 5.1 % / Rmerge(I) obs: 0.757 / Mean I/σ(I) obs: 1.8 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4P5I Resolution: 2.9→47.44 Å / Cor.coef. Fo:Fc: 0.903 / Cor.coef. Fo:Fc free: 0.837 / SU B: 41.375 / SU ML: 0.353 / Cross valid method: THROUGHOUT / ESU R: 0.492 / ESU R Free: 0.372 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 78.862 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→47.44 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj
