[English] 日本語
 Yorodumi
Yorodumi- PDB-1vwq: STREPTAVIDIN-CYCLO-[5-S-VALERAMIDE-HPQGPPC]K-NH2, PH 2.5, I4122 C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1vwq | ||||||
|---|---|---|---|---|---|---|---|
| Title | STREPTAVIDIN-CYCLO-[5-S-VALERAMIDE-HPQGPPC]K-NH2, PH 2.5, I4122 COMPLEX | ||||||
 Components Components |
| ||||||
 Keywords Keywords | COMPLEX (BIOTIN-BINDING PROTEIN/PEPTIDE) / COMPLEX (BIOTIN-BINDING PROTEIN-PEPTIDE) / CYCLIC PEPTIDE DISCOVERED BY PHAGE DISPLAY / COMPLEX (BIOTIN-BINDING PROTEIN-PEPTIDE) complex | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |   Streptomyces avidinii (bacteria) Streptomyces avidinii (bacteria)  Bothrops insularis (golden lancehead) Bothrops insularis (golden lancehead) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.7 Å X-RAY DIFFRACTION / Resolution: 1.7 Å | ||||||
 Authors Authors | Katz, B.A. / Cass, R.T. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 1997 Journal: J.Biol.Chem. / Year: 1997Title: In crystals of complexes of streptavidin with peptide ligands containing the HPQ sequence the pKa of the peptide histidine is less than 3.0. Authors: Katz, B.A. / Cass, R.T. #1:  Journal: J.Am.Chem.Soc. / Year: 1996 Journal: J.Am.Chem.Soc. / Year: 1996Title: Structure-Based Design Tools: Structural and Thermodynamic Comparison with Biotin of a Small Molecule that Binds Streptavidin with Micromolar Affinity Authors: Katz, B.A. / Liu, B. / Cass, R.T. #2:  Journal: J.Am.Chem.Soc. / Year: 1996 Journal: J.Am.Chem.Soc. / Year: 1996Title: Preparation of a Protein-Dimerizing Ligand by Topochemistry and Structure-Based Design Authors: Katz, B.A. #3:  Journal: J.Biol.Chem. / Year: 1995 Journal: J.Biol.Chem. / Year: 1995Title: Topochemical Catalysis Achieved by Structure-Based Ligand Design Authors: Katz, B.A. / Cass, R.T. / Liu, B. / Arze, R. / Collins, N. #4:  Journal: Chem.Biol. / Year: 1995 Journal: Chem.Biol. / Year: 1995Title: Topochemistry for Preparing Ligands that Dimerize Receptors Authors: Katz, B.A. / Stroud, R.M. / Collins, N. / Liu, B. / Arze, R. #5:  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Binding to Protein Targets of Peptidic Leads Discovered by Phage Display: Crystal Structures of Streptavidin-Bound Linear and Cyclic Peptide Ligands Containing the Hpq Sequence Authors: Katz, B.A. #6:  Journal: J.Am.Chem.Soc. / Year: 1995 Journal: J.Am.Chem.Soc. / Year: 1995Title: Structure-Based Design of High Affinity Streptavidin Binding Cyclic Peptide Ligands Containing Thioether Cross-Links Authors: Katz, B.A. / Johnson, C.R. / Cass, R.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1vwq.cif.gz 1vwq.cif.gz | 62.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1vwq.ent.gz pdb1vwq.ent.gz | 52.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1vwq.json.gz 1vwq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vw/1vwq https://data.pdbj.org/pub/pdb/validation_reports/vw/1vwq ftp://data.pdbj.org/pub/pdb/validation_reports/vw/1vwq ftp://data.pdbj.org/pub/pdb/validation_reports/vw/1vwq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1vwaC  1vwbC  1vwcC  1vwdC  1vweC  1vwfC  1vwgC  1vwhC  1vwiC  1vwjC  1vwkC  1vwlC  1vwmC  1vwnC  1vwoC  1vwpC  1vwrC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein |  Mass: 12965.025 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Streptomyces avidinii (bacteria) / References: UniProt: P22629 Streptomyces avidinii (bacteria) / References: UniProt: P22629 |
|---|---|
| #2: Protein/peptide | Mass: 863.017 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Bothrops insularis (golden lancehead) Bothrops insularis (golden lancehead) |
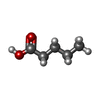
| #3: Chemical | ChemComp-LEA /  Valeric acid Valeric acid |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.66 Å3/Da / Density % sol: 33.3 % | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 2.5 Details: SYNTHETIC MOTHER LIQUOR = 75 % SATURATED AMMONIUM SULFATE, 25 % 1.0 M SODIUM FORMATE ADJUSTED TO PH 2.5. | |||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 4.5 / Method: vapor diffusion, hanging drop / Details: Pahler, A., (1987) J. Biol. Chem., 262, 13933. | |||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Num. obs: 28160 / Redundancy: 4.5 % / Rmerge(I) obs: 0.107 |
| Reflection | *PLUS Highest resolution: 1.33 Å / Num. measured all: 127962 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.7→7.5 Å / σ(F): 2.5 Details: THE FOLLOWING ATOMS HAD WEAK DENSITY AND OCCUPANCIES WERE REFINED: 13, 14, 15, (MAIN CHAIN OF GLN 24), (SIDE CHAIN OF GLN 24), (N, HN, CA, HA1, HA2 OF GLY 26), (MAIN CHAIN OF ALA 35), (SIDE ...Details: THE FOLLOWING ATOMS HAD WEAK DENSITY AND OCCUPANCIES WERE REFINED: 13, 14, 15, (MAIN CHAIN OF GLN 24), (SIDE CHAIN OF GLN 24), (N, HN, CA, HA1, HA2 OF GLY 26), (MAIN CHAIN OF ALA 35), (SIDE CHAIN OF ALA 35), (MAIN CHAIN AND CB, HB1, HB2 OF ASP 36), (CG, OD1, AND OD2 OF ASP 36), 46, 47, 48, 49, 50, (MAIN CHAIN AND CB, HB1, HB2 OF GLU 51), (CG, HG1, HG2, CD, OE1, OE2 OF GLU 51), (SIDE CHAIN OF SER 52), (TERMINUS OF ARG 53), 99, 100, (N AND HN OF GLU 101), (CG, HG1, HG2, CD, OE1, OE2 OF GLU 101), TERMINUS OF ARG 103, (N, HN, CA, HA OF GLU 116), (CB, HB1, HB2, CG, HG1, HG2 OF GLU 116), (CD, OE1, OE2 OF GLU 116), (N, HN, CA, HA OF ALA 117), (CB, HB1, HB2, HB3 OF ALA 117), LYS 121 FROM CG OUTWARD, LYS 132 SIDE CHAIN, (LYS P 9 AND NH2 P 10). DISCRETELY DISORDERED ENTIRE RESIDUES WHOSE OCCUPANCIES AND STRUCTURES WERE SIMULTANEOUSLY REFINED ARE: 60, 61, 62, 63, 64, 65, 66, 67, 68, 69. DISCRETELY DISORDERED SIDE CHAINS WHOSE OCCUPANCIES AND STRUCTURES WERE SIMULTANEOUSLY REFINED ARE: ARG 84, GLN 107. RESIDUES 60 - 69 WERE REFINED IN 2 CONFORMATIONS BECAUSE UPON PROTONATION OF ASP 61 AT LOW PH, ASP 61 UNDERGOES A LARGE SHIFT IN CONFORMATION AND CHANGE IN HYDROGEN BONDING. THE LOOP COMPRISING RESIDUES 61 - 69 ALSO UNDERGO CORRESPONDING CONFORMATIONAL CHANGES. HOWEVER SOME OF THESE RESIDUES ARE DISORDERED AND NOT VISIBLE IN EITHER CONFORMATION. DISORDERED WATERS ARE HOH 166 WHICH IS CLOSE TO A SYMMETRY-RELATED EQUIVALENT OF HOH 354; HOH 148 WHICH OCCUPIES THE SPACE AVAILABLE WHEN ASP 61 IS IN CONFORMATION 2; HOH 279, HOH 305, HOH 313, HOH 516, HOH 526, HOH 532 WHICH ARE CLOSE TO SYMMETRY-RELATED EQUIVALENTS OF THEMSELVES; NO ENERGY INTERACTIONS INVOLVING HOH 279, HOH 305, HOH 313, HOH 516, HOH 526, HOH 532 WERE TURNED ON DURING REFINEMENT. THE FOLLOWING ATOMS HAD WEAK DENSITY AND OCCUPANCIES WERE REFINED: 13, 14, 15, (MAIN CHAIN OF GLN 24), (SIDE CHAIN OF GLN 24), (N, HN, CA, HA1, HA2 OF GLY 26), (MAIN CHAIN OF ALA 35), (SIDE CHAIN OF ALA 35), (MAIN CHAIN AND CB, HB1, HB2 OF ASP 36), (CG, OD1, AND OD2 OF ASP 36), 46, 47, 48, 49, 50, (MAIN CHAIN AND CB, HB1, HB2 OF GLU 51), (CG, HG1, HG2, CD, OE1, OE2 OF GLU 51), (SIDE CHAIN OF SER 52), (TERMINUS OF ARG 53), 99, 100, (N AND HN OF GLU 101), (CG, HG1, HG2, CD, OE1, OE2 OF GLU 101), TERMINUS OF ARG 103, (N, HN, CA, HA OF GLU 116), (CB, HB1, HB2, CG, HG1, HG2 OF GLU 116), (CD, OE1, OE2 OF GLU 116), (N, HN, CA, HA OF ALA 117), (CB, HB1, HB2, HB3 OF ALA 117), LYS 121 FROM CG OUTWARD, LYS 132 SIDE CHAIN, (LYS P 9 AND NH2 P 10). RESIDUES 60 - 69 WERE REFINED IN 2 CONFORMATIONS BECAUSE UPON PROTONATION OF ASP 61 AT LOW PH, ASP 61 UNDERGOES A LARGE SHIFT IN CONFORMATION AND CHANGE IN HYDROGEN BONDING. THE LOOP COMPRISING RESIDUES 61 - 69 ALSO UNDERGO CORRESPONDING CONFORMATIONAL CHANGES. HOWEVER SOME OF THESE RESIDUES ARE DISORDERED AND NOT VISIBLE IN EITHER CONFORMATION.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→7.5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.7→1.78 Å / % reflection obs: 60.5 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj