+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gvg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Clavaminate Synthase with Nitric Oxide | ||||||
 Components Components | CLAVAMINATE SYNTHASE 1 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / LYASE / OXYGENASE / TRIFUNCTIONAL ENZYME / CLAVAMINATE SYNTHASE 1 / JELLY ROLL / NITRIC OXIDE | ||||||
| Function / homology |  Function and homology information Function and homology informationclavaminate synthase / clavaminate synthase activity / clavulanic acid biosynthetic process / antibiotic biosynthetic process / iron ion binding Similarity search - Function | ||||||
| Biological species |  STREPTOMYCES CLAVULIGERUS (bacteria) STREPTOMYCES CLAVULIGERUS (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.54 Å MOLECULAR REPLACEMENT / Resolution: 1.54 Å | ||||||
 Authors Authors | Zhang, Z.H. / Ren, J. / McKinnon, C.H. / Clifton, I.J. / Harlos, K. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: FEBS Lett. / Year: 2002 Journal: FEBS Lett. / Year: 2002Title: Crystal Structure of a Clavaminate Synthase-Fe(II) -2-Oxoglutarate-Substrate-No Complex: Evidence for Metal Centered Rearrangements Authors: Zhang, Z.H. / Ren, J. / Harlos, K. / McKinnon, C.H. / Clifton, I.J. / Schofield, C.J. #1:  Journal: Nat.Struct.Biol. / Year: 2000 Journal: Nat.Struct.Biol. / Year: 2000Title: Structural Origins of the Selectivity of the Trifunctional Oxygenase Clavaminic Acid Synthase Authors: Zhang, Z.H. / Ren, J. / Stammers, D.K. / Baldwin, J.E. / Harlos, K. / Schofield, C.J. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN ... SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gvg.cif.gz 1gvg.cif.gz | 86.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gvg.ent.gz pdb1gvg.ent.gz | 62.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gvg.json.gz 1gvg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gv/1gvg https://data.pdbj.org/pub/pdb/validation_reports/gv/1gvg ftp://data.pdbj.org/pub/pdb/validation_reports/gv/1gvg ftp://data.pdbj.org/pub/pdb/validation_reports/gv/1gvg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ds0S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 35415.785 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  STREPTOMYCES CLAVULIGERUS (bacteria) / Plasmid: PET11A / Production host: STREPTOMYCES CLAVULIGERUS (bacteria) / Plasmid: PET11A / Production host:  |
|---|
-Non-polymers , 6 types, 399 molecules 


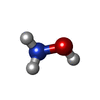






| #2: Chemical | ChemComp-FE / | ||||||
|---|---|---|---|---|---|---|---|
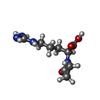
| #3: Chemical | ChemComp-AKG / | ||||||
| #4: Chemical | | #5: Chemical | ChemComp-PCX / | #6: Chemical | ChemComp-HOA / | #7: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 43.65 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 7.6 / Details: pH 7.60 | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 7.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200H / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200H / Wavelength: 1.5418 |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: Feb 15, 2001 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.54→30 Å / Num. obs: 46381 / % possible obs: 97.7 % / Redundancy: 13.2 % / Biso Wilson estimate: 19.8 Å2 / Rmerge(I) obs: 0.054 / Net I/σ(I): 29.1 |
| Reflection shell | Resolution: 1.54→1.6 Å / Redundancy: 4.46 % / Rmerge(I) obs: 0.298 / Mean I/σ(I) obs: 3.8 / % possible all: 86 |
| Reflection | *PLUS Lowest resolution: 30 Å / Num. measured all: 301822 |
| Reflection shell | *PLUS % possible obs: 86 % / Num. unique obs: 4038 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1DS0 Resolution: 1.54→30 Å / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 46.29 Å2 / ksol: 0.4 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.54→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 30 Å / % reflection Rfree: 5 % / Rfactor Rfree: 0.2074 / Rfactor Rwork: 0.1813 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj






