[English] 日本語
 Yorodumi
Yorodumi- PDB-1e4y: Mutant P9L of adenylate kinase from E. coli, modified in the Gly-loop -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1e4y | ||||||
|---|---|---|---|---|---|---|---|
| Title | Mutant P9L of adenylate kinase from E. coli, modified in the Gly-loop | ||||||
 Components Components | Adenylate kinase | ||||||
 Keywords Keywords | TRANSFERASE(PHOSPHOTRANSFERASE) | ||||||
| Function / homology |  Function and homology information Function and homology informationpurine ribonucleotide interconversion / ADP biosynthetic process / nucleoside monophosphate metabolic process / nucleoside diphosphate metabolic process /  adenylate kinase / adenylate kinase /  adenylate kinase activity / AMP salvage / adenylate kinase activity / AMP salvage /  nucleoside diphosphate kinase activity / AMP binding / nucleoside diphosphate kinase activity / AMP binding /  phosphorylation ...purine ribonucleotide interconversion / ADP biosynthetic process / nucleoside monophosphate metabolic process / nucleoside diphosphate metabolic process / phosphorylation ...purine ribonucleotide interconversion / ADP biosynthetic process / nucleoside monophosphate metabolic process / nucleoside diphosphate metabolic process /  adenylate kinase / adenylate kinase /  adenylate kinase activity / AMP salvage / adenylate kinase activity / AMP salvage /  nucleoside diphosphate kinase activity / AMP binding / nucleoside diphosphate kinase activity / AMP binding /  phosphorylation / magnesium ion binding / phosphorylation / magnesium ion binding /  ATP binding / ATP binding /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | ||||||
 Authors Authors | Mueller, C.W. / Schulz, G.E. | ||||||
 Citation Citation |  Journal: Proteins / Year: 1993 Journal: Proteins / Year: 1993Title: Crystal structures of two mutants of adenylate kinase from Escherichia coli that modify the Gly-loop. Authors: Muller, C.W. / Schulz, G.E. #1: Journal: J.Mol.Biol. / Year: 1990 Title: Induced-Fit Movements in Adenylate Kinases Authors: Schulz, G.E. / Mueller, C.W. / Diederichs, K. #2: Journal: J.Mol.Biol. / Year: 1988 Title: Structure of the Complex of Adenylate Kinase from Escherichia Coli with the Inhibitor P1, P5-Bis (Adenosine-5'-) Pentaphosphate Authors: Mueller, C.W. / Schulz, G.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1e4y.cif.gz 1e4y.cif.gz | 89.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1e4y.ent.gz pdb1e4y.ent.gz | 74.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1e4y.json.gz 1e4y.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e4/1e4y https://data.pdbj.org/pub/pdb/validation_reports/e4/1e4y ftp://data.pdbj.org/pub/pdb/validation_reports/e4/1e4y ftp://data.pdbj.org/pub/pdb/validation_reports/e4/1e4y | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 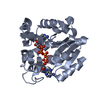
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.995341, 0.068333, 0.068023), Vector  : : |
- Components
Components
| #1: Protein |  / AK / ATP-AMP transphosphorylase / ATP:AMP phosphotransferase / Adenylate monophosphate kinase / AK / ATP-AMP transphosphorylase / ATP:AMP phosphotransferase / Adenylate monophosphate kinaseMass: 23678.150 Da / Num. of mol.: 2 / Mutation: L9P Source method: isolated from a genetically manipulated source Details: AP5A / Source: (gene. exp.)   Escherichia coli (E. coli) / Strain: CV2 / Gene: adk, dnaW, plsA, b0474, JW0463 / Production host: Escherichia coli (E. coli) / Strain: CV2 / Gene: adk, dnaW, plsA, b0474, JW0463 / Production host:   Escherichia coli (E. coli) / References: UniProt: P69441, Escherichia coli (E. coli) / References: UniProt: P69441,  adenylate kinase adenylate kinase#2: Chemical | Compound details | CHAIN A, B ENGINEERED | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.85 Å3/Da / Density % sol: 56.84 % | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 7.2 / Details: pH 7.20 | ||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 6.7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 279 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 3.4→10 Å / Num. obs: 7261 / % possible obs: 90 % / Observed criterion σ(I): 0 / Redundancy: 2 % / Rmerge(I) obs: 0.1 / Rsym value: 0.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 1.85→10 Å / σ(F): 0 MOLECULAR REPLACEMENT / Resolution: 1.85→10 Å / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj