[English] 日本語
 Yorodumi
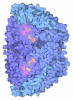
Yorodumi- PDB-9qr1: Methyl-coenzyme M reductase of ANME-2d Candidatus Methanoperedens... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9qr1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Methyl-coenzyme M reductase of ANME-2d Candidatus Methanoperedens sp. BLZ2 from a bioreactor enrichment culture | ||||||||||||||||||
 Components Components |
| ||||||||||||||||||
 Keywords Keywords | TRANSFERASE / methanotrophy / anaerobic methanotrophic archaea / methane oxidation / ANME / methyl-coenzyme M reductase / MCR / reverse methanogenesis / nickel-dependent enzyme / coenzyme M / coenzyme B / F430 cofactor | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcoenzyme-B sulfoethylthiotransferase / coenzyme-B sulfoethylthiotransferase activity / methanogenesis / metal ion binding Similarity search - Function | ||||||||||||||||||
| Biological species |  Candidatus Methanoperedens sp. BLZ2 (archaea) Candidatus Methanoperedens sp. BLZ2 (archaea) | ||||||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 0.98 Å MOLECULAR REPLACEMENT / Resolution: 0.98 Å | ||||||||||||||||||
 Authors Authors | Mueller, M.-C. / Wagner, T. | ||||||||||||||||||
| Funding support | European Union,  Germany, Germany,  Netherlands, 5items Netherlands, 5items
| ||||||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Atomic resolution structures of the key enzyme MCR in anaerobic methanotrophy reveal novel and extensive post-translational modifications. Authors: Mueller, M.-C. / Wissink, M. / Mukherjee, P. / von Possel, N. / Laso-Perez, R. / Engilberge, S. / Carpentier, P. / Kahnt, J. / Wegener, G. / Welte, C.U. / Wagner, T. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9qr1.cif.gz 9qr1.cif.gz | 1.5 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9qr1.ent.gz pdb9qr1.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  9qr1.json.gz 9qr1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9qr1_validation.pdf.gz 9qr1_validation.pdf.gz | 854.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9qr1_full_validation.pdf.gz 9qr1_full_validation.pdf.gz | 859.8 KB | Display | |
| Data in XML |  9qr1_validation.xml.gz 9qr1_validation.xml.gz | 142.2 KB | Display | |
| Data in CIF |  9qr1_validation.cif.gz 9qr1_validation.cif.gz | 203.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qr/9qr1 https://data.pdbj.org/pub/pdb/validation_reports/qr/9qr1 ftp://data.pdbj.org/pub/pdb/validation_reports/qr/9qr1 ftp://data.pdbj.org/pub/pdb/validation_reports/qr/9qr1 | HTTPS FTP |
-Related structure data
| Related structure data |  9qr3C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Methyl-coenzyme M reductase subunit ... , 2 types, 4 molecules ADBE
| #1: Protein | Mass: 61535.535 Da / Num. of mol.: 2 / Source method: isolated from a natural source Details: The alpha subunit contains six post-translational modifications: Methylhistidine 268 Methylarginine 282 Hydroxytryptophane 439 Thioglycine 457 Didehydroaspartate 462 Methylcysteine 464 Source: (natural)  Candidatus Methanoperedens sp. BLZ2 (archaea) Candidatus Methanoperedens sp. BLZ2 (archaea)Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: BLZ2 / Tissue: / References: UniProt: A0A6A2FLY3, coenzyme-B sulfoethylthiotransferase #2: Protein | Mass: 45366.742 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Candidatus Methanoperedens sp. BLZ2 (archaea) Candidatus Methanoperedens sp. BLZ2 (archaea)Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: BLZ2 / Tissue: / References: UniProt: A0A6A2G3Q8, coenzyme-B sulfoethylthiotransferase |
|---|
-Protein , 1 types, 2 molecules CF
| #3: Protein | Mass: 28197.822 Da / Num. of mol.: 2 / Source method: isolated from a natural source Details: The gamma subunit contains a methylhistidine at position 159. Source: (natural)  Candidatus Methanoperedens sp. BLZ2 (archaea) Candidatus Methanoperedens sp. BLZ2 (archaea)Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: BLZ2 / Tissue: / References: UniProt: A0A5E4HJB0, coenzyme-B sulfoethylthiotransferase |
|---|
-Non-polymers , 8 types, 3454 molecules 











| #4: Chemical | ChemComp-NA / | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #5: Chemical | ChemComp-EDO / #6: Chemical | ChemComp-NO3 / #7: Chemical | #8: Chemical | #9: Chemical | #10: Chemical | #11: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 44 % / Description: Thick, yellow, brick-shaped |
|---|---|
| Crystal grow | Temperature: 291.15 K / Method: vapor diffusion, sitting drop / pH: 8 Details: Crystallisation was carried out on a junior Clover plate using a solution containing 20% w/v polyethylene glycol 3,350, 50 mM Tris pH 8.0, and 200 mM potassium nitrate. The reservoir ...Details: Crystallisation was carried out on a junior Clover plate using a solution containing 20% w/v polyethylene glycol 3,350, 50 mM Tris pH 8.0, and 200 mM potassium nitrate. The reservoir contained 100 ul of crystallisation solution. 5 ul protein at 2.19 mg/ml in 25 mM Tris/HCl pH 8.0, 100 mM NaCl, 10% v/v glycerol and 2 mM dithiothreitol were mixed with 2 ul of the crystallisation solution. Crystals were soaked in a crystallisation solution supplemented with 20% ethylene glycol before freezing in liquid nitrogen. |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM07 / Wavelength: 0.9798 Å / Beamline: BM07 / Wavelength: 0.9798 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Mar 30, 2023 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9798 Å / Relative weight: 1 |
| Reflection | Resolution: 0.976→76.674 Å / Num. obs: 1130261 / % possible obs: 96.5 % / Redundancy: 7.8 % / CC1/2: 0.996 / Rmerge(I) obs: 0.119 / Rpim(I) all: 0.045 / Rrim(I) all: 0.128 / Net I/σ(I): 9.4 |
| Reflection shell | Resolution: 0.976→1.04 Å / Redundancy: 7.1 % / Rmerge(I) obs: 1.168 / Mean I/σ(I) obs: 1.7 / Num. unique obs: 56513 / CC1/2: 0.667 / Rpim(I) all: 0.468 / Rrim(I) all: 1.26 / % possible all: 70.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 0.98→41.78 Å / SU ML: 0.06 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 10.72 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 0.98→41.78 Å / SU ML: 0.06 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 10.72 / Stereochemistry target values: MLDetails: The model was built and corrected with COOT (Version 0.8.9.2)(52) and refined with PHENIX.refine. All atoms were considered anisotropic, with hydrogens added in riding mode. The model was ...Details: The model was built and corrected with COOT (Version 0.8.9.2)(52) and refined with PHENIX.refine. All atoms were considered anisotropic, with hydrogens added in riding mode. The model was validated by the MolProbity server (http://molprobity.biochem.duke.edu).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 10.72 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 0.98→41.78 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj