+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9nh8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CHD1-nucleosome complex (anchored state) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | nuclear protein/DNA / chromatin / remodeler / genome organization / nuclear protein / nuclear protein-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationnucleosome organization / ATP-dependent chromatin remodeler activity / histone H3K4me3 reader activity / host-mediated activation of viral transcription / nuclear chromosome / helicase activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of chromatin / heterochromatin formation / nucleosome ...nucleosome organization / ATP-dependent chromatin remodeler activity / histone H3K4me3 reader activity / host-mediated activation of viral transcription / nuclear chromosome / helicase activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of chromatin / heterochromatin formation / nucleosome / nucleosome assembly / histone binding / Estrogen-dependent gene expression / chromatin remodeling / protein heterodimerization activity / chromatin binding / chromatin / ATP hydrolysis activity / DNA binding / nucleoplasm / ATP binding / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species | synthetic construct (others)  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | James, A.M. / Farnung, L. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Mol Cell / Year: 2025 Journal: Mol Cell / Year: 2025Title: Structural basis of human CHD1 nucleosome recruitment and pausing. Authors: Allison M James / Lucas Farnung /  Abstract: Chromatin remodelers regulate gene expression and genome maintenance by controlling nucleosome positioning, but the structural basis for their regulated and directional activity remains poorly ...Chromatin remodelers regulate gene expression and genome maintenance by controlling nucleosome positioning, but the structural basis for their regulated and directional activity remains poorly understood. Here, we present three cryoelectron microscopy (cryo-EM) structures of human chromodomain helicase DNA-binding protein 1 (CHD1) bound to nucleosomes that reveal previously unobserved recruitment and regulatory states. We identify a structural element, termed the "anchor element," that connects the CHD1 ATPase motor to the nucleosome entry-side acidic patch. The anchor element coordinates with other regulatory modules, including the gating element, which undergoes a conformational switch critical for remodeling. Our structures demonstrate how the DNA-binding region of CHD1 binds entry- and exit-side DNA during remodeling to achieve directional sliding. The observed structural elements are conserved across chromatin remodelers, suggesting a unified mechanism for nucleosome recognition and remodeling. Our findings show how chromatin remodelers couple nucleosome recruitment to regulated DNA translocation, providing a framework for understanding chromatin remodeler mechanisms beyond DNA translocation. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9nh8.cif.gz 9nh8.cif.gz | 494.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9nh8.ent.gz pdb9nh8.ent.gz | 373.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9nh8.json.gz 9nh8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nh/9nh8 https://data.pdbj.org/pub/pdb/validation_reports/nh/9nh8 ftp://data.pdbj.org/pub/pdb/validation_reports/nh/9nh8 ftp://data.pdbj.org/pub/pdb/validation_reports/nh/9nh8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  49406MC  9earC  42693 M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 5 types, 10 molecules AEVBFCGDHW
| #1: Protein | Mass: 15463.181 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Details: C110A / Source: (gene. exp.)  #2: Protein | Mass: 11394.426 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  #3: Protein | Mass: 14109.436 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  #4: Protein | Mass: 13655.948 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  #7: Protein | | Mass: 151787.688 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CHD1 / Production host: Homo sapiens (human) / Gene: CHD1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: O14646, DNA helicase Trichoplusia ni (cabbage looper) / References: UniProt: O14646, DNA helicase |
|---|
-DNA chain , 2 types, 2 molecules IJ
| #5: DNA chain | Mass: 63146.293 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host: synthetic construct (others) |
|---|---|
| #6: DNA chain | Mass: 63434.418 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host: synthetic construct (others) |
-Non-polymers , 1 types, 1 molecules 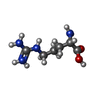
| #8: Chemical | ChemComp-ARG / |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: CHD1-nucleosome complex / Type: COMPLEX / Entity ID: #1-#7 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.4 MDa / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 54.6 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 67512 / Symmetry type: POINT |
 Movie
Movie Controller
Controller








 PDBj
PDBj








































