[English] 日本語
 Yorodumi
Yorodumi- PDB-9kvl: Neutron and X-ray joint refined structure of a copper-containing ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9kvl | ||||||
|---|---|---|---|---|---|---|---|
| Title | Neutron and X-ray joint refined structure of a copper-containing nitrite reductase (C135A mutant) in complex with nitrite | ||||||
 Components Components | Copper-containing nitrite reductase | ||||||
 Keywords Keywords | OXIDOREDUCTASE / copper / denitrification | ||||||
| Function / homology |  Function and homology information Function and homology informationnitrite reductase (NO-forming) / nitrite reductase (NO-forming) activity / copper ion binding Similarity search - Function | ||||||
| Biological species |  Geobacillus thermodenitrificans NG80-2 (bacteria) Geobacillus thermodenitrificans NG80-2 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / NEUTRON DIFFRACTION / X-RAY DIFFRACTION / NEUTRON DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.7 Å MOLECULAR REPLACEMENT / Resolution: 1.7 Å | ||||||
 Authors Authors | Fukuda, Y. / Lintuluoto, M. / Hirano, Y. / Kusaka, K. / Inoue, T. / Tamada, T. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2025 Journal: J.Biol.Chem. / Year: 2025Title: Structural basis of cuproenzyme nitrite reduction at the level of a single hydrogen atom. Authors: Fukuda, Y. / Lintuluoto, M. / Hirano, Y. / Kusaka, K. / Inoue, T. / Tamada, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9kvl.cif.gz 9kvl.cif.gz | 260.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9kvl.ent.gz pdb9kvl.ent.gz | 207.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9kvl.json.gz 9kvl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kv/9kvl https://data.pdbj.org/pub/pdb/validation_reports/kv/9kvl ftp://data.pdbj.org/pub/pdb/validation_reports/kv/9kvl ftp://data.pdbj.org/pub/pdb/validation_reports/kv/9kvl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9kvmC  9kwsC  9kwtC  9kwuC  9kwvC  4ysoS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||
| Unit cell |
| ||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 33071.500 Da / Num. of mol.: 1 / Mutation: C135A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Geobacillus thermodenitrificans NG80-2 (bacteria) Geobacillus thermodenitrificans NG80-2 (bacteria)Gene: nirK, GTNG_0650 / Production host:  |
|---|
-Non-polymers , 5 types, 342 molecules 

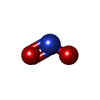






| #2: Chemical | ChemComp-MPD / ( | ||
|---|---|---|---|
| #3: Chemical | ChemComp-MRD / ( | ||
| #4: Chemical | ChemComp-NO2 / | ||
| #5: Chemical | | #6: Chemical | ChemComp-DOD / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment |
|
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.03 Å3/Da / Density % sol: 59.44 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 4.5 Details: 0.1 M acetate buffer pH 4.5, 5.5% (w/v) PEG 4000, and 75 mM CuSO4 |
-Data collection
| Diffraction |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||||||||||||||
| Detector |
| |||||||||||||||||||||||||||
| Radiation |
| |||||||||||||||||||||||||||
| Radiation wavelength |
| |||||||||||||||||||||||||||
| Reflection | Biso Wilson estimate: 6.43 Å2 / Entry-ID: 9KVL
| |||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | SU ML: 0.11 / Cross valid method: FREE R-VALUE / Method to determine structure:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→13.2 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj











