[English] 日本語
 Yorodumi
Yorodumi- PDB-9hk5: Structure of a mutant of human protein kinase CK2alpha' that equa... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9hk5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of a mutant of human protein kinase CK2alpha' that equals its isoenzyme CK2alpha in affinity to the regulatory subunit CK2beta | ||||||
 Components Components | Casein kinase II subunit alpha' | ||||||
 Keywords Keywords | TRANSFERASE / human protein kinase CK2 human casein kinase 2 isoenzymes CK2alpha and CK2alpha' CK2alpha/CK2beta interaction | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of mitophagy / regulation of chromosome separation / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes / protein kinase CK2 complex / : / Receptor Mediated Mitophagy / Synthesis of PC / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Maturation of hRSV A proteins ...regulation of mitophagy / regulation of chromosome separation / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes / protein kinase CK2 complex / : / Receptor Mediated Mitophagy / Synthesis of PC / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Maturation of hRSV A proteins / negative regulation of apoptotic signaling pathway / negative regulation of proteasomal ubiquitin-dependent protein catabolic process / liver regeneration / acrosomal vesicle / Signal transduction by L1 / cerebral cortex development / Wnt signaling pathway / Regulation of PTEN stability and activity / KEAP1-NFE2L2 pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / double-strand break repair / spermatogenesis / Regulation of TP53 Activity through Phosphorylation / non-specific serine/threonine protein kinase / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / DNA damage response / chromatin / nucleoplasm / ATP binding / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.491 Å MOLECULAR REPLACEMENT / Resolution: 1.491 Å | ||||||
 Authors Authors | Werner, C. / Niefind, K. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Biol.Chem. / Year: 2025 Journal: Biol.Chem. / Year: 2025Title: A CK2 alpha ' mutant indicating why CK2 alpha and CK2 alpha ', the isoforms of the catalytic subunit of human protein kinase CK2, deviate in affinity to CK2 beta. Authors: Werner, C. / Eimermacher, S. / Harasimowicz, H. / Fischer, D. / Pietsch, M. / Niefind, K. #1:  Journal: J Mol Biol / Year: 2011 Journal: J Mol Biol / Year: 2011Title: Structure of the human protein kinase CK2 catalytic subunit CK2alpha' and interaction thermodynamics with the regulatory subunit CK2beta Authors: Bischoff, N. / Olsen, B. / Raaf, J. / Bretner, M. / Issinger, O.G. / Niefind, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9hk5.cif.gz 9hk5.cif.gz | 190.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9hk5.ent.gz pdb9hk5.ent.gz | 122.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9hk5.json.gz 9hk5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9hk5_validation.pdf.gz 9hk5_validation.pdf.gz | 804.4 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9hk5_full_validation.pdf.gz 9hk5_full_validation.pdf.gz | 805.7 KB | Display | |
| Data in XML |  9hk5_validation.xml.gz 9hk5_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  9hk5_validation.cif.gz 9hk5_validation.cif.gz | 28.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hk/9hk5 https://data.pdbj.org/pub/pdb/validation_reports/hk/9hk5 ftp://data.pdbj.org/pub/pdb/validation_reports/hk/9hk5 ftp://data.pdbj.org/pub/pdb/validation_reports/hk/9hk5 | HTTPS FTP |
-Related structure data
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 42907.965 Da / Num. of mol.: 1 / Mutation: P32V, V83I, T101I, C336S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CSNK2A2, CK2A2 / Production host: Homo sapiens (human) / Gene: CSNK2A2, CK2A2 / Production host:  References: UniProt: P19784, non-specific serine/threonine protein kinase | ||||||||
|---|---|---|---|---|---|---|---|---|---|
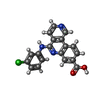
| #2: Chemical | | #3: Chemical | ChemComp-3NG / | #4: Water | ChemComp-HOH / | Has ligand of interest | N | Has protein modification | N | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.45 Å3/Da / Density % sol: 49.75 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: protein stock solution: 5 mg/ml protein, 500 mM NaCl, 25 mM Tris/HCl, pH 8.5; protein/inhibitor mixture: 19 mikroliter protein stock solution plus 1 mikroliter 20 mM CX-4945 in DMSO; ...Details: protein stock solution: 5 mg/ml protein, 500 mM NaCl, 25 mM Tris/HCl, pH 8.5; protein/inhibitor mixture: 19 mikroliter protein stock solution plus 1 mikroliter 20 mM CX-4945 in DMSO; reservoir: 900 mM LiCL, 28 % (w/v) PEG 6000, 250 mM Tris/HCl, pH 8.5; crystallization drop: 2 mikroliter reservoir + 4 mikroliter protein/inhibitor mixture; initial crystals were optimized by micro seeding |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID30B / Wavelength: 0.873128 Å / Beamline: ID30B / Wavelength: 0.873128 Å |
| Detector | Type: DECTRIS EIGER2 X 9M / Detector: PIXEL / Date: Nov 28, 2024 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873128 Å / Relative weight: 1 |
| Reflection | Resolution: 1.491→46.971 Å / Num. obs: 44234 / % possible obs: 88.3 % / Redundancy: 2.8 % / Biso Wilson estimate: 9.63 Å2 / CC1/2: 0.992 / Rmerge(I) obs: 0.104 / Rpim(I) all: 0.076 / Rrim(I) all: 0.129 / Rsym value: 0.104 / Net I/σ(I): 7.7 |
| Reflection shell | Resolution: 1.491→1.672 Å / Redundancy: 3 % / Rmerge(I) obs: 0.775 / Mean I/σ(I) obs: 1.6 / Num. unique obs: 2189 / CC1/2: 0.447 / Rpim(I) all: 0.533 / Rrim(I) all: 0.944 / Rsym value: 0.775 / % possible all: 11.4 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.491→46.97 Å / SU ML: 0.1324 / Cross valid method: FREE R-VALUE / σ(F): 1.96 / Phase error: 23.1756 MOLECULAR REPLACEMENT / Resolution: 1.491→46.97 Å / SU ML: 0.1324 / Cross valid method: FREE R-VALUE / σ(F): 1.96 / Phase error: 23.1756 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.8 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.491→46.97 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION / Auth asym-ID: A / Label asym-ID: A
|
 Movie
Movie Controller
Controller


 PDBj
PDBj