[English] 日本語
 Yorodumi
Yorodumi- PDB-9fka: Cryo-EM structure of the reduced cytochrome bd oxidase from M. tu... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9fka | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the reduced cytochrome bd oxidase from M. tuberculosis | ||||||
 Components Components | (Probable integral membrane cytochrome D ubiquinol oxidase (Subunit ...) x 2 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / Ubiquinone / Demethylmenaquinone / Cryo-EM / Respiration / Substrate specificity / Disulfide regulation | ||||||
| Function / homology |  Function and homology information Function and homology informationcytochrome complex / aerobic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / electron transfer activity / heme binding / metal ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.96 Å | ||||||
 Authors Authors | Kayastha, K. / Bruenle, S. | ||||||
| Funding support |  Netherlands, 1items Netherlands, 1items
| ||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2025 Journal: J Biol Chem / Year: 2025Title: Menaquinone-specific turnover by Mycobacterium tuberculosis cytochrome bd is redox regulated by the Q-loop disulfide bond. Authors: Tijn T van der Velden / Kanwal Kayastha / Caspar Y J Waterham / Steffen Brünle / Lars J C Jeuken /  Abstract: Cytochrome bd from Mycobacterium tuberculosis (Mtbd) is a menaquinol oxidase that has gained interest as an antibiotic target because of its importance in survival under infectious conditions. Mtbd ...Cytochrome bd from Mycobacterium tuberculosis (Mtbd) is a menaquinol oxidase that has gained interest as an antibiotic target because of its importance in survival under infectious conditions. Mtbd contains a characteristic disulfide bond that has been hypothesized to allow for Mtbd activity regulation at the enzymatic level, possibly helping M. tuberculosis to rapidly adapt to the hostile environment of the phagosome. Here, the role of the disulfide bond and quinone specificity have been determined by reconstitution of a minimal respiratory chain and the single-particle cryo-EM structure in the disulfide-reduced form. Mtbd was shown to be specific for menaquinone, while regulation by reduction of the Q-loop disulfide bond decreased oxidase activity up to 90%. Structural analysis shows that a salt bridge unique to Mtbd keeps the Q-loop partially structured in its disulfide-reduced form, which could facilitate the rapid activation of Mtbd upon exposure to reactive oxygen species. We signify Mtbd as the first redox sensory terminal oxidase and propose that this helps M. tuberculosis in the defense against reactive oxygen species encountered during infection. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9fka.cif.gz 9fka.cif.gz | 188 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9fka.ent.gz pdb9fka.ent.gz | 139.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9fka.json.gz 9fka.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9fka_validation.pdf.gz 9fka_validation.pdf.gz | 1.6 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9fka_full_validation.pdf.gz 9fka_full_validation.pdf.gz | 1.6 MB | Display | |
| Data in XML |  9fka_validation.xml.gz 9fka_validation.xml.gz | 43.2 KB | Display | |
| Data in CIF |  9fka_validation.cif.gz 9fka_validation.cif.gz | 60.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fk/9fka https://data.pdbj.org/pub/pdb/validation_reports/fk/9fka ftp://data.pdbj.org/pub/pdb/validation_reports/fk/9fka ftp://data.pdbj.org/pub/pdb/validation_reports/fk/9fka | HTTPS FTP |
-Related structure data
| Related structure data |  50520MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Probable integral membrane cytochrome D ubiquinol oxidase (Subunit ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 53863.098 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria)Gene: cydA, Rv1623c / Production host:  Mycolicibacterium smegmatis (bacteria) / References: UniProt: L7N662 Mycolicibacterium smegmatis (bacteria) / References: UniProt: L7N662 |
|---|---|
| #2: Protein | Mass: 37650.957 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria)Gene: cydB, Rv1622c / Production host:  Mycolicibacterium smegmatis (bacteria) / References: UniProt: O06139 Mycolicibacterium smegmatis (bacteria) / References: UniProt: O06139 |
-Non-polymers , 6 types, 7 molecules 
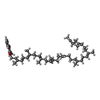









| #3: Chemical | ChemComp-PTY / | ||||||
|---|---|---|---|---|---|---|---|
| #4: Chemical | ChemComp-MQ9 / | ||||||
| #5: Chemical | | #6: Chemical | ChemComp-HDD / | #7: Chemical | ChemComp-CDL / | #8: Chemical | ChemComp-CD4 / ( | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cytochrome BD-I oxidase / Type: COMPLEX Details: Cytochrome BD-I oxidase bound with Heme B, cis-Heme D, Menaquinone-9, Cardiolipins, PE lipid Entity ID: #1-#2 / Source: RECOMBINANT | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.942 MDa / Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||||
| Source (recombinant) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) | ||||||||||||||||||||
| Buffer solution | pH: 7.4 / Details: 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.005% LMNG | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 1.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||
| Specimen support | Details: 15 mAmp / Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: C-flat-1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK III / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 105000 X / Nominal defocus max: 2400 nm / Nominal defocus min: 1000 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 4.04 sec. / Electron dose: 100 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 2 / Num. of real images: 14078 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Details: Objective aperture of 100 um / Energyfilter slit width: 20 eV |
| Image scans | Sampling size: 10 µm |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 15300000 | ||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.96 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 1277019 / Algorithm: BACK PROJECTION / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: BACKBONE TRACE / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 7NKZ Accession code: 7NKZ / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Highest resolution: 2.96 Å Stereochemistry target values: REAL-SPACE (WEIGHTED MAP SUM AT ATOM CENTERS) | ||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj







