[English] 日本語
 Yorodumi
Yorodumi- EMDB-50520: Cryo-EM structure of the reduced cytochrome bd oxidase from M. tu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the reduced cytochrome bd oxidase from M. tuberculosis | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ubiquinone / Demethylmenaquinone / Cryo-EM / Respiration / Substrate specificity / Disulfide regulation / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytochrome complex / aerobic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / electron transfer activity / heme binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.96 Å | |||||||||
 Authors Authors | Kayastha K / Bruenle S | |||||||||
| Funding support |  Netherlands, 1 items Netherlands, 1 items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2025 Journal: J Biol Chem / Year: 2025Title: Menaquinone-specific turnover by Mycobacterium tuberculosis cytochrome bd is redox regulated by the Q-loop disulfide bond. Authors: Tijn T van der Velden / Kanwal Kayastha / Caspar Y J Waterham / Steffen Brünle / Lars J C Jeuken /  Abstract: Cytochrome bd from Mycobacterium tuberculosis (Mtbd) is a menaquinol oxidase that has gained interest as an antibiotic target because of its importance in survival under infectious conditions. Mtbd ...Cytochrome bd from Mycobacterium tuberculosis (Mtbd) is a menaquinol oxidase that has gained interest as an antibiotic target because of its importance in survival under infectious conditions. Mtbd contains a characteristic disulfide bond that has been hypothesized to allow for Mtbd activity regulation at the enzymatic level, possibly helping M. tuberculosis to rapidly adapt to the hostile environment of the phagosome. Here, the role of the disulfide bond and quinone specificity have been determined by reconstitution of a minimal respiratory chain and the single-particle cryo-EM structure in the disulfide-reduced form. Mtbd was shown to be specific for menaquinone, while regulation by reduction of the Q-loop disulfide bond decreased oxidase activity up to 90%. Structural analysis shows that a salt bridge unique to Mtbd keeps the Q-loop partially structured in its disulfide-reduced form, which could facilitate the rapid activation of Mtbd upon exposure to reactive oxygen species. We signify Mtbd as the first redox sensory terminal oxidase and propose that this helps M. tuberculosis in the defense against reactive oxygen species encountered during infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50520.map.gz emd_50520.map.gz | 31.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50520-v30.xml emd-50520-v30.xml emd-50520.xml emd-50520.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50520_fsc.xml emd_50520_fsc.xml | 11.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_50520.png emd_50520.png | 148.2 KB | ||
| Masks |  emd_50520_msk_1.map emd_50520_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-50520.cif.gz emd-50520.cif.gz | 7.5 KB | ||
| Others |  emd_50520_half_map_1.map.gz emd_50520_half_map_1.map.gz emd_50520_half_map_2.map.gz emd_50520_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50520 http://ftp.pdbj.org/pub/emdb/structures/EMD-50520 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50520 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50520 | HTTPS FTP |
-Validation report
| Summary document |  emd_50520_validation.pdf.gz emd_50520_validation.pdf.gz | 731.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50520_full_validation.pdf.gz emd_50520_full_validation.pdf.gz | 730.8 KB | Display | |
| Data in XML |  emd_50520_validation.xml.gz emd_50520_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  emd_50520_validation.cif.gz emd_50520_validation.cif.gz | 21.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50520 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50520 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50520 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50520 | HTTPS FTP |
-Related structure data
| Related structure data |  9fkaMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50520.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50520.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.836 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_50520_msk_1.map emd_50520_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_50520_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_50520_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cytochrome BD-I oxidase
| Entire | Name: Cytochrome BD-I oxidase |
|---|---|
| Components |
|
-Supramolecule #1: Cytochrome BD-I oxidase
| Supramolecule | Name: Cytochrome BD-I oxidase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Cytochrome BD-I oxidase bound with Heme B, cis-Heme D, Menaquinone-9, Cardiolipins, PE lipid |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 942 KDa |
-Macromolecule #1: Probable integral membrane cytochrome D ubiquinol oxidase (Subuni...
| Macromolecule | Name: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit I) CydA (Cytochrome BD-I oxidase subunit I) type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
| Molecular weight | Theoretical: 53.863098 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MNVVDISRWQ FGITTVYHFI FVPLTIGLAP LIAVMQTLWV VTDNPAWYRL TKFFGKLFLI NFAIGVATGI VQEFQFGMNW SEYSRFVGD VFGAPLAMEG LAAFFFESTF IGLWIFGWNR LPRLVHLACI WIVAIAVNVS AFFIIAANSF MQHPVGAHYN P TTGRAELS ...String: MNVVDISRWQ FGITTVYHFI FVPLTIGLAP LIAVMQTLWV VTDNPAWYRL TKFFGKLFLI NFAIGVATGI VQEFQFGMNW SEYSRFVGD VFGAPLAMEG LAAFFFESTF IGLWIFGWNR LPRLVHLACI WIVAIAVNVS AFFIIAANSF MQHPVGAHYN P TTGRAELS SIVVLLTNNT AQAAFTHTVS GALLTAGTFV AAVSAWWLVR SSTTHADSDT QAMYRPATIL GCWVALAATA GL LFTGDHQ GKLMFQQQPM KMASAESLCD TQTDPNFSVL TVGRQNNCDS LTRVIEVPYV LPFLAEGRIS GVTLQGIRDL QQE YQQRFG PNDYRPNLFV TYWSFRMMIG LMAIPVLFAL IALWLTRGGQ IPNQRWFSWL ALLTMPAPFL ANSAGWVFTE MGRQ PWVVV PNPTGDQLVR LTVKAGVSDH SATVVATSLL MFTLVYAVLA VIWCWLLKRY IVEGPLEHDA EPAAHGAPRD DEVAP LSFA Y UniProtKB: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit I) CydA (Cytochrome BD-I oxidase subunit I) |
-Macromolecule #2: Probable integral membrane cytochrome D ubiquinol oxidase (Subuni...
| Macromolecule | Name: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II) type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
| Molecular weight | Theoretical: 37.650957 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MVLQELWFGV IAALFLGFFI LEGFDFGVGM LMAPFAHVGM GDPETHRRTA LNTIGPVWDG NEVWLITAGA AIFAAFPGWY ATVFSALYL PLLAILFGMI LRAVAIEWRG KIDDPKWRTG ADFGIAAGSW LPALLWGVAF AILVRGLPVD ANGHVALSIP D VLNAYTLL ...String: MVLQELWFGV IAALFLGFFI LEGFDFGVGM LMAPFAHVGM GDPETHRRTA LNTIGPVWDG NEVWLITAGA AIFAAFPGWY ATVFSALYL PLLAILFGMI LRAVAIEWRG KIDDPKWRTG ADFGIAAGSW LPALLWGVAF AILVRGLPVD ANGHVALSIP D VLNAYTLL GGLATAGLFS LYGAVFIALK TSGPIRDDAY RFAVWLSLPV AGLVAGFGLW TQLAYGKDWT WLVLAVAGCA QA AATVLVW RRVSDGWAFM CTLIVVAAVV VLLFGALYPN LVPSTLNPQW SLTIHNASST PYTLKIMTWV TAFFAPLTVA YQT WTYWVF RQRISAERIP PPTGLARRAP UniProtKB: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II) |
-Macromolecule #3: PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 3 / Number of copies: 1 / Formula: PTY |
|---|---|
| Molecular weight | Theoretical: 734.039 Da |
| Chemical component information |  ChemComp-PTY: |
-Macromolecule #4: MENAQUINONE-9
| Macromolecule | Name: MENAQUINONE-9 / type: ligand / ID: 4 / Number of copies: 1 / Formula: MQ9 |
|---|---|
| Molecular weight | Theoretical: 785.233 Da |
| Chemical component information | 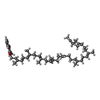 ChemComp-MQ9: |
-Macromolecule #5: PROTOPORPHYRIN IX CONTAINING FE
| Macromolecule | Name: PROTOPORPHYRIN IX CONTAINING FE / type: ligand / ID: 5 / Number of copies: 2 / Formula: HEM |
|---|---|
| Molecular weight | Theoretical: 616.487 Da |
| Chemical component information |  ChemComp-HEM: |
-Macromolecule #6: CIS-HEME D HYDROXYCHLORIN GAMMA-SPIROLACTONE
| Macromolecule | Name: CIS-HEME D HYDROXYCHLORIN GAMMA-SPIROLACTONE / type: ligand / ID: 6 / Number of copies: 1 / Formula: HDD |
|---|---|
| Molecular weight | Theoretical: 632.487 Da |
| Chemical component information |  ChemComp-HDD: |
-Macromolecule #7: CARDIOLIPIN
| Macromolecule | Name: CARDIOLIPIN / type: ligand / ID: 7 / Number of copies: 1 / Formula: CDL |
|---|---|
| Molecular weight | Theoretical: 1.464043 KDa |
| Chemical component information |  ChemComp-CDL: |
-Macromolecule #8: (2R,5R,11R,14R)-5,8,11-trihydroxy-5,11-dioxido-17-oxo-2,14-bis(te...
| Macromolecule | Name: (2R,5R,11R,14R)-5,8,11-trihydroxy-5,11-dioxido-17-oxo-2,14-bis(tetradecanoyloxy)-4,6,10,12,16-pentaoxa-5,11-diphosphatriacont-1-yl tetradecanoate type: ligand / ID: 8 / Number of copies: 1 / Formula: CD4 |
|---|---|
| Molecular weight | Theoretical: 1.241633 KDa |
| Chemical component information |  ChemComp-CD4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.005% LMNG | ||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 90 sec. / Pretreatment - Atmosphere: OTHER / Details: 15 mAmp | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV / Details: Objective aperture of 100 um |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 2 / Number real images: 14078 / Average exposure time: 4.04 sec. / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)














































