[English] 日本語
 Yorodumi
Yorodumi- PDB-9e3e: Torpedo muscle-type nicotinic acetylcholine receptor - Diliganded... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9e3e | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Torpedo muscle-type nicotinic acetylcholine receptor - Diliganded State | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components | (Acetylcholine receptor subunit ...) x 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Nicotinic acetylcholine receptor / Ion channel / Cys-loop receptor / Pentameric ligand-gated ion channel / Neurotransmitter-gated receptor / Ligand-gated ion channel / Primed state | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationacetylcholine-gated monoatomic cation-selective channel activity / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / transmembrane signaling receptor activity / postsynaptic membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Thompson, M.J. / Nury, H. / Zarkadas, E. / Baenziger, J.E. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Canada, European Union, 6items Canada, European Union, 6items
| |||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2026 Journal: Science / Year: 2026Title: Asynchronous subunit transitions prime acetylcholine receptor activation. Authors: Mackenzie J Thompson / Christian J G Tessier / Anna Ananchenko / Camille Hénault / Johnathon R Emlaw / François Dehez / Eleftherios Zarkadas / Corrie J B daCosta / Hugues Nury / John E Baenziger /   Abstract: Communication at synapses is facilitated by postsynaptic receptors, which convert a chemical signal into an electrical response. For ligand-gated ion channels, agonist binding triggers rapid ...Communication at synapses is facilitated by postsynaptic receptors, which convert a chemical signal into an electrical response. For ligand-gated ion channels, agonist binding triggers rapid transitions through intermediate states leading to a transient open-pore conformation, with these transitions shaping the postsynaptic response. In this work, we determine structures of the muscle-type nicotinic acetylcholine receptor in unliganded, mono-liganded, and di-liganded states. Agonist binding to a single site stabilizes a closed structure where an entire principal agonist-binding subunit transitions to an active-like conformation, whereas the other unoccupied principal subunit remains inactive, albeit poised for activation. Uniting this intermediate structure with single-channel recordings informs a sequential activation mechanism where asynchronous subunit transitions prime the receptor for activation-a finding with implications for an entire superfamily of pentameric ligand-gated ion channels. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9e3e.cif.gz 9e3e.cif.gz | 526.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9e3e.ent.gz pdb9e3e.ent.gz | 343.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9e3e.json.gz 9e3e.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e3/9e3e https://data.pdbj.org/pub/pdb/validation_reports/e3/9e3e ftp://data.pdbj.org/pub/pdb/validation_reports/e3/9e3e ftp://data.pdbj.org/pub/pdb/validation_reports/e3/9e3e | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  47480MC  9e3fC  9e3gC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Acetylcholine receptor subunit ... , 4 types, 5 molecules ADBCE
| #1: Protein | Mass: 50168.164 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P02710 #2: Protein | | Mass: 53731.773 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P02712 #3: Protein | | Mass: 57625.711 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P02718 #4: Protein | | Mass: 56206.574 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P02714 |
|---|
-Sugars , 4 types, 7 molecules
| #5: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose-(1-6)-[alpha-D- ...alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source | ||||
|---|---|---|---|---|---|
| #6: Polysaccharide | Source method: isolated from a genetically manipulated source #7: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #8: Polysaccharide | Source method: isolated from a genetically manipulated source |
-Non-polymers , 1 types, 2 molecules 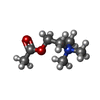
| #9: Chemical |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Torpedo muscle-type nicotinic acetylcholine receptor / Type: COMPLEX / Entity ID: #1-#4 / Source: NATURAL | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.25 MDa / Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 0.65 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Sample was monodisperce but suffered from orientation bias. | ||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2200 nm / Nominal defocus min: 700 nm / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Electron dose: 42 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 4133568 | ||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 552159 / Algorithm: FOURIER SPACE / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 155.3 Å2 | ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj


