[English] 日本語
 Yorodumi
Yorodumi- PDB-9c10: AMG 193, a clinical stage MTA-cooperative PRMT5 inhibitor, drives... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9c10 | ||||||
|---|---|---|---|---|---|---|---|
| Title | AMG 193, a clinical stage MTA-cooperative PRMT5 inhibitor, drives anti-tumor activity preclinically and in patients with MTAP-deleted cancers | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / PRMT5 / MTA-cooperative inhibitor / TRANSFERASE / TRANSFERASE-TRANSFERASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / histone H4R3 methyltransferase activity ...positive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / histone H4R3 methyltransferase activity / : / epithelial cell proliferation involved in prostate gland development / protein-arginine N-methyltransferase activity / methylosome / positive regulation of mRNA splicing, via spliceosome / methyl-CpG binding / endothelial cell activation / histone H3 methyltransferase activity / histone methyltransferase activity / regulation of mitotic nuclear division / negative regulation of gene expression via chromosomal CpG island methylation / Cul4B-RING E3 ubiquitin ligase complex / E-box binding / histone methyltransferase complex / positive regulation of oligodendrocyte differentiation / negative regulation of cell differentiation / spliceosomal snRNP assembly / ribonucleoprotein complex binding / regulation of ERK1 and ERK2 cascade / ubiquitin-like ligase-substrate adaptor activity / liver regeneration / regulation of signal transduction by p53 class mediator / methyltransferase activity / circadian regulation of gene expression / DNA-templated transcription termination / Regulation of TP53 Activity through Methylation / RMTs methylate histone arginines / protein polyubiquitination / p53 binding / transcription corepressor activity / snRNP Assembly / ubiquitin-dependent protein catabolic process / transcription coactivator activity / chromatin remodeling / protein heterodimerization activity / positive regulation of cell population proliferation / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / chromatin / Golgi apparatus / nucleoplasm / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.85 Å MOLECULAR REPLACEMENT / Resolution: 2.85 Å | ||||||
 Authors Authors | Ghimire-Rijal, S. / Mukund, S. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Cancer Discov / Year: 2025 Journal: Cancer Discov / Year: 2025Title: AMG 193, a Clinical Stage MTA-Cooperative PRMT5 Inhibitor, Drives Antitumor Activity Preclinically and in Patients with MTAP-Deleted Cancers. Authors: Belmontes, B. / Slemmons, K.K. / Su, C. / Liu, S. / Policheni, A.N. / Moriguchi, J. / Tan, H. / Xie, F. / Aiello, D.A. / Yang, Y. / Lazaro, R. / Aeffner, F. / Rees, M.G. / Ronan, M.M. / ...Authors: Belmontes, B. / Slemmons, K.K. / Su, C. / Liu, S. / Policheni, A.N. / Moriguchi, J. / Tan, H. / Xie, F. / Aiello, D.A. / Yang, Y. / Lazaro, R. / Aeffner, F. / Rees, M.G. / Ronan, M.M. / Roth, J.A. / Vestergaard, M. / Cowland, S. / Andersson, J. / Sarvary, I. / Chen, Q. / Sharma, P. / Lopez, P. / Tamayo, N. / Pettus, L.H. / Ghimire-Rijal, S. / Mukund, S. / Allen, J.R. / DeVoss, J. / Coxon, A. / Rodon, J. / Ghiringhelli, F. / Penel, N. / Prenen, H. / Glad, S. / Chuang, C.H. / Keyvanjah, K. / Townsley, D.M. / Butler, J.R. / Bourbeau, M.P. / Caenepeel, S. / Hughes, P.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9c10.cif.gz 9c10.cif.gz | 206.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9c10.ent.gz pdb9c10.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  9c10.json.gz 9c10.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9c10_validation.pdf.gz 9c10_validation.pdf.gz | 1011.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9c10_full_validation.pdf.gz 9c10_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  9c10_validation.xml.gz 9c10_validation.xml.gz | 41.8 KB | Display | |
| Data in CIF |  9c10_validation.cif.gz 9c10_validation.cif.gz | 54.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c1/9c10 https://data.pdbj.org/pub/pdb/validation_reports/c1/9c10 ftp://data.pdbj.org/pub/pdb/validation_reports/c1/9c10 ftp://data.pdbj.org/pub/pdb/validation_reports/c1/9c10 | HTTPS FTP |
-Related structure data
| Related structure data |  4gqbS S: Starting model for refinement |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 74281.250 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host: Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host:  unidentified baculovirus unidentified baculovirusReferences: UniProt: O14744, type II protein arginine methyltransferase |
|---|---|
| #2: Protein | Mass: 36883.332 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host: Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host:  unidentified baculovirus / References: UniProt: Q9BQA1 unidentified baculovirus / References: UniProt: Q9BQA1 |
-Non-polymers , 5 types, 52 molecules 

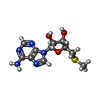




| #3: Chemical | | #4: Chemical | ChemComp-DMS / | #5: Chemical | ChemComp-A1ATH / ( | Mass: 444.406 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C22H19F3N4O3 / Feature type: SUBJECT OF INVESTIGATION #6: Chemical | ChemComp-MTA / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.87 Å3/Da / Density % sol: 57.17 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion Details: 0.1M Na citrate tribasic dihydrate pH 5.6 2% Tascimate pH 5.0, 16% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 31, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.85→41.32 Å / Num. obs: 30217 / % possible obs: 99.8 % / Redundancy: 12.6 % / CC1/2: 0.999 / Rmerge(I) obs: 0.076 / Net I/σ(I): 24.6 |
| Reflection shell | Resolution: 2.85→2.95 Å / Rmerge(I) obs: 0.076 / Mean I/σ(I) obs: 4.9 / Num. unique obs: 30217 / CC1/2: 0.999 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4GQB Resolution: 2.85→41.32 Å / SU ML: 0.4 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 36.37 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.85→41.32 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj



