[English] 日本語
 Yorodumi
Yorodumi- PDB-9ayf: Structure of human calcium-sensing receptor in complex with Gi1 (... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9ayf | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of human calcium-sensing receptor in complex with Gi1 (miniGi1) protein in detergent | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / Calcium-sensing receptor / G-protein-coupled receptor / G protein / signal transduction | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of presynaptic membrane potential / bile acid secretion / chemosensory behavior / response to fibroblast growth factor / cellular response to peptide / cellular response to vitamin D / Class C/3 (Metabotropic glutamate/pheromone receptors) / positive regulation of positive chemotaxis / fat pad development / cellular response to hepatocyte growth factor stimulus ...regulation of presynaptic membrane potential / bile acid secretion / chemosensory behavior / response to fibroblast growth factor / cellular response to peptide / cellular response to vitamin D / Class C/3 (Metabotropic glutamate/pheromone receptors) / positive regulation of positive chemotaxis / fat pad development / cellular response to hepatocyte growth factor stimulus / amino acid binding / branching morphogenesis of an epithelial tube / positive regulation of calcium ion import / positive regulation of NLRP3 inflammasome complex assembly / regulation of calcium ion transport / positive regulation of vasoconstriction / cellular response to low-density lipoprotein particle stimulus / anatomical structure morphogenesis / detection of calcium ion / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / JNK cascade / response to prostaglandin E / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / axon terminus / cellular response to forskolin / ossification / chloride transmembrane transport / regulation of mitotic spindle organization / response to ischemia / Regulation of insulin secretion / cellular response to glucose stimulus / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / positive regulation of insulin secretion / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / G protein-coupled receptor activity / response to peptide hormone / integrin binding / vasodilation / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / intracellular calcium ion homeostasis / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / GDP binding / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / G protein activity / GTPase binding / presynaptic membrane / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / cellular response to hypoxia Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||
 Authors Authors | Zuo, H. / Park, J. / Frangaj, A. / Ye, J. / Lu, G. / Manning, J.J. / Asher, W.B. / Lu, Z. / Hu, G. / Wang, L. ...Zuo, H. / Park, J. / Frangaj, A. / Ye, J. / Lu, G. / Manning, J.J. / Asher, W.B. / Lu, Z. / Hu, G. / Wang, L. / Mendez, J. / Eng, E. / Zhang, Z. / Lin, X. / Grasucci, R. / Hendrickson, W.A. / Clarke, O.B. / Javitch, J.A. / Conigrave, A.D. / Fan, Q.R. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Promiscuous G-protein activation by the calcium-sensing receptor. Authors: Hao Zuo / Jinseo Park / Aurel Frangaj / Jianxiang Ye / Guanqi Lu / Jamie J Manning / Wesley B Asher / Zhengyuan Lu / Guo-Bin Hu / Liguo Wang / Joshua Mendez / Edward Eng / Zhening Zhang / ...Authors: Hao Zuo / Jinseo Park / Aurel Frangaj / Jianxiang Ye / Guanqi Lu / Jamie J Manning / Wesley B Asher / Zhengyuan Lu / Guo-Bin Hu / Liguo Wang / Joshua Mendez / Edward Eng / Zhening Zhang / Xin Lin / Robert Grassucci / Wayne A Hendrickson / Oliver B Clarke / Jonathan A Javitch / Arthur D Conigrave / Qing R Fan /   Abstract: The human calcium-sensing receptor (CaSR) detects fluctuations in the extracellular Ca concentration and maintains Ca homeostasis. It also mediates diverse cellular processes not associated with Ca ...The human calcium-sensing receptor (CaSR) detects fluctuations in the extracellular Ca concentration and maintains Ca homeostasis. It also mediates diverse cellular processes not associated with Ca balance. The functional pleiotropy of CaSR arises in part from its ability to signal through several G-protein subtypes. We determined structures of CaSR in complex with G proteins from three different subfamilies: G, G and G. We found that the homodimeric CaSR of each complex couples to a single G protein through a common mode. This involves the C-terminal helix of each Gα subunit binding to a shallow pocket that is formed in one CaSR subunit by all three intracellular loops (ICL1-ICL3), an extended transmembrane helix 3 and an ordered C-terminal region. G-protein binding expands the transmembrane dimer interface, which is further stabilized by phospholipid. The restraint imposed by the receptor dimer, in combination with ICL2, enables G-protein activation by facilitating conformational transition of Gα. We identified a single Gα residue that determines G and G versus G selectivity. The length and flexibility of ICL2 allows CaSR to bind all three Gα subtypes, thereby conferring capacity for promiscuous G-protein coupling. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9ayf.cif.gz 9ayf.cif.gz | 455.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9ayf.ent.gz pdb9ayf.ent.gz | 360.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9ayf.json.gz 9ayf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ay/9ayf https://data.pdbj.org/pub/pdb/validation_reports/ay/9ayf ftp://data.pdbj.org/pub/pdb/validation_reports/ay/9ayf ftp://data.pdbj.org/pub/pdb/validation_reports/ay/9ayf | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  43990MC  9asbC  9avgC  9avlC  9axfC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules ABG
| #3: Protein | Mass: 25985.707 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAI1 / Cell line (production host): HEK293 GnTI- / Production host: Homo sapiens (human) / Gene: GNAI1 / Cell line (production host): HEK293 GnTI- / Production host:  Homo sapiens (human) / References: UniProt: P63096 Homo sapiens (human) / References: UniProt: P63096 |
|---|---|
| #4: Protein | Mass: 38413.895 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: GNB1 construct with N-ter Flag tag inserted after the first Met. Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Cell line (production host): HEK293 GnTI- / Production host: Homo sapiens (human) / Gene: GNB1 / Cell line (production host): HEK293 GnTI- / Production host:  Homo sapiens (human) / References: UniProt: P62873 Homo sapiens (human) / References: UniProt: P62873 |
| #5: Protein | Mass: 7861.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Cell line (production host): HEK293 GnTI- / Production host: Homo sapiens (human) / Gene: GNG2 / Cell line (production host): HEK293 GnTI- / Production host:  Homo sapiens (human) / References: UniProt: P59768 Homo sapiens (human) / References: UniProt: P59768 |
-Protein / Antibody , 2 types, 3 molecules QRH
| #1: Protein | Mass: 102864.617 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: Human calcium-sensing receptor (1-903) with a Flag tag inserted after the signal peptide Source: (gene. exp.)  Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell line (production host): HEK293 GnTI- / Production host: Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell line (production host): HEK293 GnTI- / Production host:  Homo sapiens (human) / References: UniProt: P41180 Homo sapiens (human) / References: UniProt: P41180#2: Antibody | | Mass: 31870.629 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Sugars , 2 types, 10 molecules 
| #6: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #7: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 5 types, 15 molecules 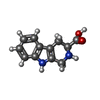








| #8: Chemical | | #9: Chemical | #10: Chemical | ChemComp-CA / #11: Chemical | #12: Chemical | ChemComp-AV0 / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human CaSR in complex with miniGi1 protein in detergent Type: COMPLEX / Entity ID: #1-#5 / Source: RECOMBINANT | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.301 MDa / Experimental value: NO | |||||||||||||||||||||||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:  Homo sapiens (human) / Cell: HEK293 GnTI- Homo sapiens (human) / Cell: HEK293 GnTI- | |||||||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | |||||||||||||||||||||||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 3.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R0.6/1 | |||||||||||||||||||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K Details: The sample was blotted for 6s before plunge-frozen. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 105000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 100 K |
| Image recording | Average exposure time: 2.5 sec. / Electron dose: 70.14 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 15713 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Width: 5760 / Height: 4092 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 3805108 | ||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 51057 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Source name: PDB / Type: experimental model
| ||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


























 PDBj
PDBj





































