+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8wrz | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cry-EM structure of cannabinoid receptor-beta-arrestin-1 complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / complex / GPCR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationrenal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / cannabinoid signaling pathway / retrograde trans-synaptic signaling by endocannabinoid / cannabinoid receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling ...renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / cannabinoid signaling pathway / retrograde trans-synaptic signaling by endocannabinoid / cannabinoid receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling / Activation of SMO / hemostasis / telencephalon development / regulation of presynaptic cytosolic calcium ion concentration / negative regulation of interleukin-8 production / G protein-coupled receptor internalization / Class A/1 (Rhodopsin-like receptors) / arrestin family protein binding / axonal fasciculation / regulation of metabolic process / Lysosome Vesicle Biogenesis / negative regulation of NF-kappaB transcription factor activity / positive regulation of cardiac muscle hypertrophy / Golgi Associated Vesicle Biogenesis / stress fiber assembly / positive regulation of Rho protein signal transduction / positive regulation of vasoconstriction / pseudopodium / positive regulation of systemic arterial blood pressure / negative regulation of interleukin-6 production / positive regulation of intracellular signal transduction / positive regulation of receptor internalization / enzyme inhibitor activity / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / negative regulation of Notch signaling pathway / endocytic vesicle / activation of adenylate cyclase activity / cellular response to hormone stimulus / Activated NOTCH1 Transmits Signal to the Nucleus / insulin-like growth factor receptor binding / clathrin-coated pit / response to cytokine / negative regulation of protein ubiquitination / GTPase activator activity / cytoplasmic vesicle membrane / clathrin-coated endocytic vesicle membrane / G protein-coupled receptor activity / electron transport chain / Signaling by high-kinase activity BRAF mutants / G protein-coupled receptor binding / MAP2K and MAPK activation / GABA-ergic synapse / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / endocytic vesicle membrane / positive regulation of protein phosphorylation / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Cargo recognition for clathrin-mediated endocytosis / Vasopressin regulates renal water homeostasis via Aquaporins / Signaling by BRAF and RAF1 fusions / Clathrin-mediated endocytosis / glucose homeostasis / protein transport / actin cytoskeleton / Thrombin signalling through proteinase activated receptors (PARs) / growth cone / presynaptic membrane / ubiquitin-dependent protein catabolic process / cytoplasmic vesicle / G alpha (i) signalling events / G alpha (s) signalling events / molecular adaptor activity / mitochondrial outer membrane / proteasome-mediated ubiquitin-dependent protein catabolic process / periplasmic space / transcription coactivator activity / electron transfer activity / positive regulation of ERK1 and ERK2 cascade / Ub-specific processing proteases / protein ubiquitination / endosome / nuclear body / G protein-coupled receptor signaling pathway / membrane raft / iron ion binding / Golgi membrane / negative regulation of cell population proliferation / lysosomal membrane / positive regulation of cell population proliferation / ubiquitin protein ligase binding / heme binding / positive regulation of gene expression / regulation of transcription by RNA polymerase II / chromatin / perinuclear region of cytoplasm / glutamatergic synapse / endoplasmic reticulum Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)Phage display vector pTDisp (others)  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Wang, Y.X. / Wang, T. / Wu, L.J. / Hua, T. / Liu, Z.J. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  China, 5items China, 5items
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Protein Cell / Year: 2024 Journal: Protein Cell / Year: 2024Title: Cryo-EM structure of cannabinoid receptor CB1-β-arrestin complex. Authors: Yuxia Wang / Lijie Wu / Tian Wang / Junlin Liu / Fei Li / Longquan Jiang / Zhongbo Fan / Yanan Yu / Na Chen / Qianqian Sun / Qiwen Tan / Tian Hua / Zhi-Jie Liu /  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8wrz.cif.gz 8wrz.cif.gz | 176.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8wrz.ent.gz pdb8wrz.ent.gz | 131.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8wrz.json.gz 8wrz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8wrz_validation.pdf.gz 8wrz_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8wrz_full_validation.pdf.gz 8wrz_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  8wrz_validation.xml.gz 8wrz_validation.xml.gz | 38.1 KB | Display | |
| Data in CIF |  8wrz_validation.cif.gz 8wrz_validation.cif.gz | 55.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz https://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz ftp://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz ftp://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz | HTTPS FTP |
-Related structure data
| Related structure data |  37795MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 44271.508 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ARRB1 / Production host: Homo sapiens (human) / Gene: ARRB1 / Production host:  |
|---|---|
| #2: Antibody | Mass: 28693.922 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) Phage display vector pTDisp (others) / Production host:  |
| #3: Protein | Mass: 53577.543 Da / Num. of mol.: 1 / Mutation: M29W, H124I, T210I, E273K, T283V, R340E Source method: isolated from a genetically manipulated source Details: The chimera of Cytochrome b-562, linker, and Cannabinoid receptor 1 Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: cybC, CNR1 / Production host:  Homo sapiens (human) / Strain (production host): CNR1 / References: UniProt: P0ABE7, UniProt: P21554 Homo sapiens (human) / Strain (production host): CNR1 / References: UniProt: P0ABE7, UniProt: P21554 |
| #4: Protein/peptide | Mass: 2920.651 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: AVPR2 / Production host: Homo sapiens (human) / Gene: AVPR2 / Production host:  |
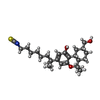
| #5: Chemical | ChemComp-8D0 / ( |
| Has ligand of interest | Y |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cry-EM structure of cannabinoid receptor-arrestin 1 complex Type: COMPLEX / Entity ID: #1-#4 / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 1.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of real images: 25847 |
- Processing
Processing
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 130058 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj