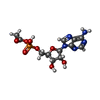
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8rwj | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryoEM structure of Acs1 filament determined by FilamentID | |||||||||
 Components Components | Acetyl-coenzyme A synthetase | |||||||||
 Keywords Keywords | CYTOSOLIC PROTEIN / metabolic enzyme / filament / cryoEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationacid-ammonia (or amide) ligase activity / acetate-CoA ligase / acetate-CoA ligase activity / acetyl-CoA biosynthetic process from acetate / AMP binding / ATP binding / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | ELECTRON MICROSCOPY / helical reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Hugener, J. / Xu, J. / Wettstein, R. / Ioannidi, L. / Velikov, D. / Wollweber, F. / Henggeler, A. / Matos, J. / Pilhofer, M. | |||||||||
| Funding support |  Switzerland, European Union, 2items Switzerland, European Union, 2items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: FilamentID reveals the composition and function of metabolic enzyme polymers during gametogenesis. Authors: Jannik Hugener / Jingwei Xu / Rahel Wettstein / Lydia Ioannidi / Daniel Velikov / Florian Wollweber / Adrian Henggeler / Joao Matos / Martin Pilhofer /   Abstract: Gamete formation and subsequent offspring development often involve extended phases of suspended cellular development or even dormancy. How cells adapt to recover and resume growth remains poorly ...Gamete formation and subsequent offspring development often involve extended phases of suspended cellular development or even dormancy. How cells adapt to recover and resume growth remains poorly understood. Here, we visualized budding yeast cells undergoing meiosis by cryo-electron tomography (cryoET) and discovered elaborate filamentous assemblies decorating the nucleus, cytoplasm, and mitochondria. To determine filament composition, we developed a "filament identification" (FilamentID) workflow that combines multiscale cryoET/cryo-electron microscopy (cryoEM) analyses of partially lysed cells or organelles. FilamentID identified the mitochondrial filaments as being composed of the conserved aldehyde dehydrogenase Ald4 and the nucleoplasmic/cytoplasmic filaments as consisting of acetyl-coenzyme A (CoA) synthetase Acs1. Structural characterization further revealed the mechanism underlying polymerization and enabled us to genetically perturb filament formation. Acs1 polymerization facilitates the recovery of chronologically aged spores and, more generally, the cell cycle re-entry of starved cells. FilamentID is broadly applicable to characterize filaments of unknown identity in diverse cellular contexts. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8rwj.cif.gz 8rwj.cif.gz | 1008.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8rwj.ent.gz pdb8rwj.ent.gz | 854.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8rwj.json.gz 8rwj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rw/8rwj https://data.pdbj.org/pub/pdb/validation_reports/rw/8rwj ftp://data.pdbj.org/pub/pdb/validation_reports/rw/8rwj ftp://data.pdbj.org/pub/pdb/validation_reports/rw/8rwj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  19548MC  8rwkC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 79270.570 Da / Num. of mol.: 9 / Source method: isolated from a natural source / Source: (natural)  #2: Chemical | ChemComp-6R9 / [[( Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method: helical reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Acetyl-CoA synthetase 1 filament from spread meiotic yeast spheroplasts Type: COMPLEX / Entity ID: #1 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  |
| Buffer solution | pH: 6.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2800 nm / Nominal defocus min: 1200 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||
| Helical symmerty | Angular rotation/subunit: 13.03 ° / Axial rise/subunit: 53.61 Å / Axial symmetry: C3 | ||||||||||||
| 3D reconstruction | Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 17169 / Symmetry type: HELICAL |
 Movie
Movie Controller
Controller







 PDBj
PDBj