+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8q1l | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | NMR structure of arthrofactin A in micellar DPC solution | |||||||||||||||
 Components Components | arthrofactin A | |||||||||||||||
 Keywords Keywords | SURFACTANT PROTEIN / Non-ribosomal polypeptide / Cyclic lipodepsipeptide / Antimicrobial peptide / Biosurfactant | |||||||||||||||
| Function / homology | polypeptide(D) / polypeptide(D) (> 10) Function and homology information Function and homology information | |||||||||||||||
| Biological species |  Pseudomonas sp. MIS38 (bacteria) Pseudomonas sp. MIS38 (bacteria) | |||||||||||||||
| Method | SOLUTION NMR / molecular dynamics | |||||||||||||||
 Authors Authors | Kovacs, B. / Geudens, N. / Martins, J.C. | |||||||||||||||
| Funding support |  Belgium, 4items Belgium, 4items
| |||||||||||||||
 Citation Citation |  Journal: Adv Sci / Year: 2025 Journal: Adv Sci / Year: 2025Title: Higher-Level Structural Classification of Pseudomonas Cyclic Lipopeptides through Their Bioactive Conformation. Authors: Kovacs, B. / Prasad, D. / De Roo, V. / Vanheede, M. / Muangkaew, P. / Madder, A. / Hofte, M. / De Mot, R. / Geudens, N. / Martins, J.C. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8q1l.cif.gz 8q1l.cif.gz | 45.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8q1l.ent.gz pdb8q1l.ent.gz | 31.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8q1l.json.gz 8q1l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q1/8q1l https://data.pdbj.org/pub/pdb/validation_reports/q1/8q1l ftp://data.pdbj.org/pub/pdb/validation_reports/q1/8q1l ftp://data.pdbj.org/pub/pdb/validation_reports/q1/8q1l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8s2aC  8s2bC  8s2cC  8s2dC  8s4lC  8s8nC  9epsC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 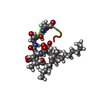
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Polypeptide(D) | Mass: 1372.646 Da / Num. of mol.: 1 / Source method: isolated from a natural source Details: 1) The polypeptide chain of arthrofactin A consists of 11 amino acids. 2) The N-terminal amino acid (D-Leu) is acylated with an (R)-3-hydroxy-decanoic acid (IG8) moeity which is indicated as ...Details: 1) The polypeptide chain of arthrofactin A consists of 11 amino acids. 2) The N-terminal amino acid (D-Leu) is acylated with an (R)-3-hydroxy-decanoic acid (IG8) moeity which is indicated as the first residue. 3) The side-chain of the D-Thr4 residue is of allo-configuration (2TL). 4) The depsi (ester) bond is established between the ASP12 carboxyl and allo-2TL side-chain OH group. Source: (natural)  Pseudomonas sp. MIS38 (bacteria) / Strain: MIS38 Pseudomonas sp. MIS38 (bacteria) / Strain: MIS38 |
|---|---|
| Has ligand of interest | Y |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Type: solution Contents: 1.3 mM NA arthrofactin A, 142.8 mM [U-2H] DPC, 90% H2O/10% D2O Details: Arthrofactin A was dissolved in the micellar solution of uniformly deuterated dodecylphosphocholine (DPC-d38). Solvent: 10 mM Na2HPO4/NaH2PO4 buffer at pH 7.4. Label: 1H / Solvent system: 90% H2O/10% D2O | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||
| Sample conditions | Ionic strength: 26 (buffer only) mM / Label: conditions_1 / pH: 7.4 / Pressure: 1 atm / Temperature: 310 K |
-NMR measurement
| NMR spectrometer | Type: Bruker AVANCE II / Manufacturer: Bruker / Model: AVANCE II / Field strength: 700 MHz / Details: Prodigy Cryoprobe |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: molecular dynamics / Software ordinal: 1 Details: The lowest energy NMR structure issued from CNS was refined in an unrestrained AMBER molecular dynamics simulation (agains the ff14SB force field). Here, we modelled the interaction of a ...Details: The lowest energy NMR structure issued from CNS was refined in an unrestrained AMBER molecular dynamics simulation (agains the ff14SB force field). Here, we modelled the interaction of a single peptide molecule with an explicit dodecylphosphocholine (DPC) micelle. The representative peptide conformation of the trajectory (=refined structure, #1) was selected using cluster analysis. Solvent model: TIP3P. Occasional too-close contacts present in the NMR structure ensemble (structures #2-#11) are fully removed during the AMBER moleculary dynamics refinement (structure #1). Side-chain outlier values for the 2TL4 residue are the results of the depsi bond. | ||||||||||||||||||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 11 |
 Movie
Movie Controller
Controller



 PDBj
PDBj HSQC
HSQC