+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8ida | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Overall structure of the LAT1-4F2hc bound with tyrosine | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN / complex / amino acid | |||||||||
| Function / homology |  Function and homology information Function and homology informationL-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity ...L-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport / methionine transport / L-leucine transmembrane transporter activity / thyroid hormone transmembrane transporter activity / isoleucine transport / valine transport / amino acid transmembrane transport / proline transport / L-amino acid transmembrane transporter activity / L-leucine transport / alanine transport / thyroid hormone transport / negative regulation of vascular associated smooth muscle cell apoptotic process / neutral amino acid transport / positive regulation of cytokine production involved in immune response / amino acid import across plasma membrane / external side of apical plasma membrane / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism / exogenous protein binding / Amino acid transport across the plasma membrane / amino acid transmembrane transporter activity / anchoring junction / antiporter activity / Basigin interactions / response to muscle activity / microvillus membrane / positive regulation of interleukin-4 production / response to exogenous dsRNA / positive regulation of interleukin-17 production / amino acid transport / tryptophan transport / positive regulation of glial cell proliferation / response to hyperoxia / xenobiotic transport / transport across blood-brain barrier / cellular response to glucose starvation / liver regeneration / negative regulation of autophagy / basal plasma membrane / peptide antigen binding / positive regulation of type II interferon production / calcium ion transport / melanosome / double-stranded RNA binding / virus receptor activity / cellular response to lipopolysaccharide / basolateral plasma membrane / carbohydrate metabolic process / apical plasma membrane / cadherin binding / intracellular membrane-bounded organelle / protein heterodimerization activity / negative regulation of gene expression / lysosomal membrane / symbiont entry into host cell / synapse / cell surface / protein homodimerization activity / RNA binding / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Yan, R.H. / Li, Y.N. / Shi, T.H. | |||||||||
| Funding support |  China, 1items China, 1items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2025 Journal: J Biol Chem / Year: 2025Title: Structural insights into the substrate transport mechanism of the amino acid transporter complex. Authors: Haonan Yang / Tianhao Shi / Jing Dong / Ting Zhang / Yaning Li / Yingying Guo / Yafei Yuan / Liuqing Yang / Jin-Tang Dong / Renhong Yan /  Abstract: The -type amino acid transporter 1 (LAT1), in complex with its ancillary protein 4F2hc, mediates the sodium-independent antiport of large neutral amino acids across the plasma membrane. LAT1 ...The -type amino acid transporter 1 (LAT1), in complex with its ancillary protein 4F2hc, mediates the sodium-independent antiport of large neutral amino acids across the plasma membrane. LAT1 preferentially transports substrates, such as -leucine, -tyrosine, and -tryptophan, thyroid hormones, and drugs like 3,4-dihydroxyphenylalanine. Its pivotal role in cancer development and progression has established LAT1 as a promising therapeutic target. While prior studies have resolved the LAT1-4F2hc architecture and inhibitor interactions, the molecular basis of LAT1 substrate selectivity remains elusive. Here, we present the cryo-EM structures of LAT1-4F2hc bound to -tyrosine, -tryptophan, -leucine, and 3,4-dihydroxyphenylalanine, revealing distinct substrate binding modes. Comparative structural analysis highlights differences between LAT1 and LAT2 in substrate coordination, driven by key residues near the binding pocket that influence transport efficiency. These findings advance our mechanistic understanding of the LAT1-4F2hc complex and provide valuable insights for structure-based drug design targeting LAT1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8ida.cif.gz 8ida.cif.gz | 180.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8ida.ent.gz pdb8ida.ent.gz | 137.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8ida.json.gz 8ida.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/id/8ida https://data.pdbj.org/pub/pdb/validation_reports/id/8ida ftp://data.pdbj.org/pub/pdb/validation_reports/id/8ida ftp://data.pdbj.org/pub/pdb/validation_reports/id/8ida | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  35361MC  8j8lC  8j8mC  8x0wC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 70160.828 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLC3A2, MDU1 / Production host: Homo sapiens (human) / Gene: SLC3A2, MDU1 / Production host:  Homo sapiens (human) / References: UniProt: P08195 Homo sapiens (human) / References: UniProt: P08195 | ||||||
|---|---|---|---|---|---|---|---|
| #2: Protein | Mass: 57186.895 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLC7A5, CD98LC, LAT1, MPE16 / Production host: Homo sapiens (human) / Gene: SLC7A5, CD98LC, LAT1, MPE16 / Production host:  Homo sapiens (human) / References: UniProt: Q01650 Homo sapiens (human) / References: UniProt: Q01650 | ||||||
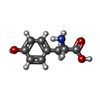
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #4: Chemical | ChemComp-TYR / | Has ligand of interest | N | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Overall structure of the LAT1-4F2hc bound with tyrosine Type: COMPLEX / Entity ID: #1-#2 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 5000 nm / Nominal defocus min: 1200 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: OTHER / Num. of particles: 170888 / Symmetry type: POINT |
 Movie
Movie Controller
Controller






 PDBj
PDBj