[English] 日本語
 Yorodumi
Yorodumi- PDB-8hvp: STRUCTURE AT 2.5-ANGSTROMS RESOLUTION OF CHEMICALLY SYNTHESIZED H... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8hvp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
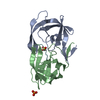
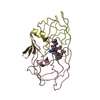
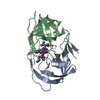
| Title | STRUCTURE AT 2.5-ANGSTROMS RESOLUTION OF CHEMICALLY SYNTHESIZED HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 PROTEASE COMPLEXED WITH A HYDROXYETHYLENE*-BASED INHIBITOR | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / ACID PROTEINASE / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | |||||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | |||||||||
 Authors Authors | Jaskolski, M. / Miller, M. / Tomasselli, A.G. / Sawyer, T.K. / Staples, D.G. / Heinrikson, R.L. / Schneider, J. / Kent, S.B.H. / Wlodawer, A. | |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 1991 Journal: Biochemistry / Year: 1991Title: Structure at 2.5-A resolution of chemically synthesized human immunodeficiency virus type 1 protease complexed with a hydroxyethylene-based inhibitor. Authors: Jaskolski, M. / Tomasselli, A.G. / Sawyer, T.K. / Staples, D.G. / Heinrikson, R.L. / Schneider, J. / Kent, S.B. / Wlodawer, A. #1:  Journal: Science / Year: 1989 Journal: Science / Year: 1989Title: Conserved Folding in Retroviral Proteases. Crystal Structure of a Synthetic HIV-1 Protease Authors: Wlodawer, A. / Miller, M. / Jaskolski, M. / Sathyanarayana, B.K. / Baldwin, E. / Weber, I.T. / Selk, L.M. / Clawson, L. / Schneider, J. / Kent, S.B.H. #2:  Journal: Science / Year: 1989 Journal: Science / Year: 1989Title: Molecular Modeling of the HIV-1 Protease and its Substrate Binding Site Authors: Weber, I.T. / Miller, M. / Jaskolski, M. / Leis, J. / Skalka, A.M. / Wlodawer, A. #3:  Journal: Nature / Year: 1989 Journal: Nature / Year: 1989Title: Crystal Structure of a Retroviral Protease Proves Relationship to Aspartic Protease Family Authors: Miller, M. / Jaskolski, M. / Rao, J.K.M. / Leis, J. / Wlodawer, A. #4:  Journal: Cell(Cambridge,Mass.) / Year: 1988 Journal: Cell(Cambridge,Mass.) / Year: 1988Title: Enzymatic Activity of a Synthetic 99 Residue Protein Corresponding to the Putative HIV-1 Protease Authors: Schneider, J. / Kent, S.B.H. | |||||||||
| History |
| |||||||||
| Remark 700 | SHEET THE DIMER INTERFACE IS COMPOSED OF INTERDIGITATED N- AND C-TERMINI FROM BOTH SUBUNITS FORMING ...SHEET THE DIMER INTERFACE IS COMPOSED OF INTERDIGITATED N- AND C-TERMINI FROM BOTH SUBUNITS FORMING A FOUR-STRANDED ANTIPARALLEL BETA-SHEET. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8hvp.cif.gz 8hvp.cif.gz | 56 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8hvp.ent.gz pdb8hvp.ent.gz | 39.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8hvp.json.gz 8hvp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hv/8hvp https://data.pdbj.org/pub/pdb/validation_reports/hv/8hvp ftp://data.pdbj.org/pub/pdb/validation_reports/hv/8hvp ftp://data.pdbj.org/pub/pdb/validation_reports/hv/8hvp | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: THE INHIBITOR RESIDUE LOV I 5 IS LEU-VAL WITH THE PEPTIDE BOND REPLACED BY CHOH-CH2. |
- Components
Components
| #1: Protein | Mass: 10764.636 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / Production host: Human immunodeficiency virus 1 / Genus: Lentivirus / Production host:  #2: Protein/peptide | | #3: Water | ChemComp-HOH / | Nonpolymer details | THE PEPTIDE BOND BETWEEN RESIDUES LEU I 5 AND VAL I 6 HAS BEEN REPLACED BY A HYDROXYETHYLENE GROUP ...THE PEPTIDE BOND BETWEEN RESIDUES LEU I 5 AND VAL I 6 HAS BEEN REPLACED BY A HYDROXYETH | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.03 % |
|---|---|
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop / Details: referred to Science 246.1149-1152 1989 |
| Components of the solutions | *PLUS Conc.: 60 % / Common name: ammonium sulfate |
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.5 Å / Num. all: 25199 / Num. obs: 6304 |
- Processing
Processing
| Software | Name: PROFFT / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→10 Å /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.5 Å / Lowest resolution: 10 Å / Num. reflection obs: 4768 / Rfactor obs: 0.138 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller



















 PDBj
PDBj




