+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8gaa | ||||||
|---|---|---|---|---|---|---|---|
| Title | C6HR1_4r: Extendable repeat protein hexamer | ||||||
 Components Components | C6HR1_4r | ||||||
 Keywords Keywords | DE NOVO PROTEIN / oligomer / repeat protein | ||||||
| Biological species | synthetic construct (others) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.24 Å | ||||||
 Authors Authors | Bethel, N.P. / Borst, A.J. / Baker, D. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nat Chem / Year: 2023 Journal: Nat Chem / Year: 2023Title: Precisely patterned nanofibres made from extendable protein multiplexes. Authors: Neville P Bethel / Andrew J Borst / Fabio Parmeggiani / Matthew J Bick / T J Brunette / Hannah Nguyen / Alex Kang / Asim K Bera / Lauren Carter / Marcos C Miranda / Ryan D Kibler / Mila Lamb ...Authors: Neville P Bethel / Andrew J Borst / Fabio Parmeggiani / Matthew J Bick / T J Brunette / Hannah Nguyen / Alex Kang / Asim K Bera / Lauren Carter / Marcos C Miranda / Ryan D Kibler / Mila Lamb / Xinting Li / Banumathi Sankaran / David Baker /   Abstract: Molecular systems with coincident cyclic and superhelical symmetry axes have considerable advantages for materials design as they can be readily lengthened or shortened by changing the length of the ...Molecular systems with coincident cyclic and superhelical symmetry axes have considerable advantages for materials design as they can be readily lengthened or shortened by changing the length of the constituent monomers. Among proteins, alpha-helical coiled coils have such symmetric, extendable architectures, but are limited by the relatively fixed geometry and flexibility of the helical protomers. Here we describe a systematic approach to generating modular and rigid repeat protein oligomers with coincident C to C and superhelical symmetry axes that can be readily extended by repeat propagation. From these building blocks, we demonstrate that a wide range of unbounded fibres can be systematically designed by introducing hydrophilic surface patches that force staggering of the monomers; the geometry of such fibres can be precisely tuned by varying the number of repeat units in the monomer and the placement of the hydrophilic patches. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8gaa.cif.gz 8gaa.cif.gz | 409.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8gaa.ent.gz pdb8gaa.ent.gz | 341 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8gaa.json.gz 8gaa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ga/8gaa https://data.pdbj.org/pub/pdb/validation_reports/ga/8gaa ftp://data.pdbj.org/pub/pdb/validation_reports/ga/8gaa ftp://data.pdbj.org/pub/pdb/validation_reports/ga/8gaa | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  29849MC  8eovC  8eoxC  8eozC  8erwC  8g8iC  8ga9C  8gaqC M: map data used to model this data C: citing same article ( |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 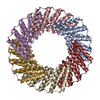
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 24246.176 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host:  Has protein modification | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: C6HR1_4r: Extendable repeat protein hexamer / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.14526 MDa / Experimental value: NO |
| Source (natural) | Organism: synthetic construct (others) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.16_3549: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.24 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 34491 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj
