[English] 日本語
 Yorodumi
Yorodumi- PDB-8e5b: Human L-type voltage-gated calcium channel Cav1.3 in the presence... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8e5b | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human L-type voltage-gated calcium channel Cav1.3 in the presence of Amiodarone and Sofosbuvir at 3.3 Angstrom resolution | |||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | TRANSPORT PROTEIN / Cav1.3 / Channels / Calcium Ion-Selective | |||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationvoltage-gated calcium channel activity involved SA node cell action potential / positive regulation of high voltage-gated calcium channel activity / voltage-gated calcium channel activity involved in cardiac muscle cell action potential / positive regulation of adenylate cyclase activity / Presynaptic depolarization and calcium channel opening / regulation of membrane repolarization during action potential / membrane depolarization during SA node cell action potential / calcium ion transmembrane transport via high voltage-gated calcium channel / regulation of atrial cardiac muscle cell membrane repolarization / high voltage-gated calcium channel activity ...voltage-gated calcium channel activity involved SA node cell action potential / positive regulation of high voltage-gated calcium channel activity / voltage-gated calcium channel activity involved in cardiac muscle cell action potential / positive regulation of adenylate cyclase activity / Presynaptic depolarization and calcium channel opening / regulation of membrane repolarization during action potential / membrane depolarization during SA node cell action potential / calcium ion transmembrane transport via high voltage-gated calcium channel / regulation of atrial cardiac muscle cell membrane repolarization / high voltage-gated calcium channel activity / membrane depolarization during bundle of His cell action potential / L-type voltage-gated calcium channel complex / membrane depolarization during cardiac muscle cell action potential / NCAM1 interactions / regulation of ventricular cardiac muscle cell membrane repolarization / positive regulation of calcium ion transport / cardiac muscle cell action potential involved in contraction / calcium ion import / regulation of potassium ion transmembrane transport / calcium ion transport into cytosol / Sensory processing of sound by inner hair cells of the cochlea / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / ankyrin binding / voltage-gated calcium channel complex / Mechanical load activates signaling by PIEZO1 and integrins in osteocytes / alpha-actinin binding / regulation of heart rate by cardiac conduction / calcium ion import across plasma membrane / regulation of calcium ion transport / neuronal dense core vesicle / voltage-gated calcium channel activity / presynaptic active zone membrane / sarcoplasmic reticulum / protein localization to plasma membrane / calcium channel regulator activity / Regulation of insulin secretion / sensory perception of sound / calcium ion transmembrane transport / GABA-ergic synapse / calcium channel activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Z disc / cellular response to amyloid-beta / Adrenaline,noradrenaline inhibits insulin secretion / calcium ion transport / T cell receptor signaling pathway / chemical synaptic transmission / synapse / extracellular exosome / metal ion binding / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Gao, S. / Yao, X. / Yan, N. | |||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  United States, 1items United States, 1items
| |||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Structural basis for the severe adverse interaction of sofosbuvir and amiodarone on L-type Ca channels. Authors: Xia Yao / Shuai Gao / Jixin Wang / Zhangqiang Li / Jian Huang / Yan Wang / Zhifei Wang / Jiaofeng Chen / Xiao Fan / Weipeng Wang / Xueqin Jin / Xiaojing Pan / Yong Yu / Armando Lagrutta / Nieng Yan /   Abstract: Drug-drug interaction of the antiviral sofosbuvir and the antiarrhythmics amiodarone has been reported to cause fatal heartbeat slowing. Sofosbuvir and its analog, MNI-1, were reported to potentiate ...Drug-drug interaction of the antiviral sofosbuvir and the antiarrhythmics amiodarone has been reported to cause fatal heartbeat slowing. Sofosbuvir and its analog, MNI-1, were reported to potentiate the inhibition of cardiomyocyte calcium handling by amiodarone, which functions as a multi-channel antagonist, and implicate its inhibitory effect on L-type Ca channels, but the molecular mechanism has remained unclear. Here we present systematic cryo-EM structural analysis of Ca1.1 and Ca1.3 treated with amiodarone or sofosbuvir alone, or sofosbuvir/MNI-1 combined with amiodarone. Whereas amiodarone alone occupies the dihydropyridine binding site, sofosbuvir is not found in the channel when applied on its own. In the presence of amiodarone, sofosbuvir/MNI-1 is anchored in the central cavity of the pore domain through specific interaction with amiodarone and directly obstructs the ion permeation path. Our study reveals the molecular basis for the physical, pharmacodynamic interaction of two drugs on the scaffold of Ca channels. | |||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8e5b.cif.gz 8e5b.cif.gz | 483 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8e5b.ent.gz pdb8e5b.ent.gz | 373.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8e5b.json.gz 8e5b.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8e5b_validation.pdf.gz 8e5b_validation.pdf.gz | 1.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8e5b_full_validation.pdf.gz 8e5b_full_validation.pdf.gz | 1.9 MB | Display | |
| Data in XML |  8e5b_validation.xml.gz 8e5b_validation.xml.gz | 74.7 KB | Display | |
| Data in CIF |  8e5b_validation.cif.gz 8e5b_validation.cif.gz | 111.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e5/8e5b https://data.pdbj.org/pub/pdb/validation_reports/e5/8e5b ftp://data.pdbj.org/pub/pdb/validation_reports/e5/8e5b ftp://data.pdbj.org/pub/pdb/validation_reports/e5/8e5b | HTTPS FTP |
-Related structure data
| Related structure data |  27909MC  8e56C  8e57C  8e58C  8e59C  8e5aC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Voltage-dependent L-type calcium channel subunit ... , 2 types, 2 molecules AC
| #1: Protein | Mass: 245417.734 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNA1D, CACH3, CACN4, CACNL1A2, CCHL1A2 / Production host: Homo sapiens (human) / Gene: CACNA1D, CACH3, CACN4, CACNL1A2, CCHL1A2 / Production host:  Homo sapiens (human) / References: UniProt: Q01668 Homo sapiens (human) / References: UniProt: Q01668 |
|---|---|
| #3: Protein | Mass: 54607.852 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNB3, CACNLB3 / Production host: Homo sapiens (human) / Gene: CACNB3, CACNLB3 / Production host:  Homo sapiens (human) / References: UniProt: P54284 Homo sapiens (human) / References: UniProt: P54284 |
-Protein , 1 types, 1 molecules D
| #2: Protein | Mass: 124692.469 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNA2D1, CACNL2A, CCHL2A, MHS3 / Production host: Homo sapiens (human) / Gene: CACNA2D1, CACNL2A, CCHL2A, MHS3 / Production host:  Homo sapiens (human) / References: UniProt: P54289 Homo sapiens (human) / References: UniProt: P54289 |
|---|
-Sugars , 4 types, 6 molecules 
| #4: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | ||||
|---|---|---|---|---|---|
| #5: Polysaccharide | Source method: isolated from a genetically manipulated source #6: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #10: Sugar | ChemComp-NAG / | |
-Non-polymers , 3 types, 4 molecules 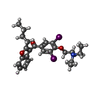
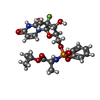



| #7: Chemical | ChemComp-BBI / ( |
|---|---|
| #8: Chemical | ChemComp-WG6 / |
| #9: Chemical |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cav1.3 / Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 281 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2100 nm / Nominal defocus min: 1900 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.2_4158: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 69718 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj


















