[English] 日本語
 Yorodumi
Yorodumi- PDB-8ayl: Resting state GluA1/A2 AMPA receptor in complex with TARP gamma 8... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8ayl | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Resting state GluA1/A2 AMPA receptor in complex with TARP gamma 8 and ligand JNJ-61432059 | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / AMPAR / ion channels / neurotransmission | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationPhase 0 - rapid depolarisation / Phase 2 - plateau phase / Cargo concentration in the ER / axonal spine / positive regulation of locomotion involved in locomotory behavior / positive regulation of membrane potential / COPII-mediated vesicle transport / cellular response to ammonium ion / response to sucrose / L-type voltage-gated calcium channel complex ...Phase 0 - rapid depolarisation / Phase 2 - plateau phase / Cargo concentration in the ER / axonal spine / positive regulation of locomotion involved in locomotory behavior / positive regulation of membrane potential / COPII-mediated vesicle transport / cellular response to ammonium ion / response to sucrose / L-type voltage-gated calcium channel complex / neuron spine / myosin V binding / postsynaptic neurotransmitter receptor diffusion trapping / proximal dendrite / regulation of AMPA receptor activity / regulation of monoatomic ion transmembrane transport / Trafficking of AMPA receptors / channel regulator activity / LGI-ADAM interactions / protein phosphatase 2B binding / response to arsenic-containing substance / cellular response to L-glutamate / cellular response to dsRNA / dendritic spine membrane / long-term synaptic depression / beta-2 adrenergic receptor binding / Synaptic adhesion-like molecules / cellular response to peptide hormone stimulus / spine synapse / response to morphine / dendritic spine neck / neuronal cell body membrane / dendritic spine head / cellular response to amine stimulus / response to psychosocial stress / peptide hormone receptor binding / spinal cord development / Activation of AMPA receptors / perisynaptic space / ligand-gated monoatomic cation channel activity / protein kinase A binding / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / transmission of nerve impulse / response to lithium ion / behavioral response to pain / kainate selective glutamate receptor activity / cellular response to glycine / AMPA glutamate receptor complex / adenylate cyclase binding / extracellularly glutamate-gated ion channel activity / immunoglobulin binding / asymmetric synapse / ionotropic glutamate receptor complex / conditioned place preference / response to electrical stimulus / regulation of receptor recycling / excitatory synapse / G-protein alpha-subunit binding / glutamate receptor binding / Unblocking of NMDA receptors, glutamate binding and activation / positive regulation of excitatory postsynaptic potential / positive regulation of synaptic transmission / long-term memory / positive regulation of synaptic transmission, glutamatergic / regulation of postsynaptic membrane neurotransmitter receptor levels / regulation of synaptic transmission, glutamatergic / postsynaptic density, intracellular component / neuronal action potential / response to fungicide / voltage-gated calcium channel activity / glutamate-gated receptor activity / synapse assembly / regulation of long-term synaptic depression / cytoskeletal protein binding / extracellular ligand-gated monoatomic ion channel activity / cellular response to brain-derived neurotrophic factor stimulus / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / somatodendritic compartment / ionotropic glutamate receptor binding / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / dendrite cytoplasm / ionotropic glutamate receptor signaling pathway / synaptic membrane / dendritic shaft / SNARE binding / PDZ domain binding / response to cocaine / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic transmission, glutamatergic / calcium channel regulator activity / protein tetramerization / cellular response to amino acid stimulus / response to nutrient levels / neuromuscular junction / establishment of protein localization / recycling endosome / postsynaptic density membrane Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Zhang, D. / Lape, R. / Shaikh, S. / Kohegyi, B. / Watson, J.F. / Cais, O. / Nakagawa, T. / Greger, I. | ||||||||||||
| Funding support |  United Kingdom, United Kingdom,  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Modulatory mechanisms of TARP γ8-selective AMPA receptor therapeutics. Authors: Danyang Zhang / Remigijus Lape / Saher A Shaikh / Bianka K Kohegyi / Jake F Watson / Ondrej Cais / Terunaga Nakagawa / Ingo H Greger /    Abstract: AMPA glutamate receptors (AMPARs) mediate excitatory neurotransmission throughout the brain. Their signalling is uniquely diversified by brain region-specific auxiliary subunits, providing an ...AMPA glutamate receptors (AMPARs) mediate excitatory neurotransmission throughout the brain. Their signalling is uniquely diversified by brain region-specific auxiliary subunits, providing an opportunity for the development of selective therapeutics. AMPARs associated with TARP γ8 are enriched in the hippocampus, and are targets of emerging anti-epileptic drugs. To understand their therapeutic activity, we determined cryo-EM structures of the GluA1/2-γ8 receptor associated with three potent, chemically diverse ligands. We find that despite sharing a lipid-exposed and water-accessible binding pocket, drug action is differentially affected by binding-site mutants. Together with patch-clamp recordings and MD simulations we also demonstrate that ligand-triggered reorganisation of the AMPAR-TARP interface contributes to modulation. Unexpectedly, one ligand (JNJ-61432059) acts bifunctionally, negatively affecting GluA1 but exerting positive modulatory action on GluA2-containing AMPARs, in a TARP stoichiometry-dependent manner. These results further illuminate the action of TARPs, demonstrate the sensitive balance between positive and negative modulatory action, and provide a mechanistic platform for development of both positive and negative selective AMPAR modulators. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8ayl.cif.gz 8ayl.cif.gz | 712.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8ayl.ent.gz pdb8ayl.ent.gz | 559.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8ayl.json.gz 8ayl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ay/8ayl https://data.pdbj.org/pub/pdb/validation_reports/ay/8ayl ftp://data.pdbj.org/pub/pdb/validation_reports/ay/8ayl ftp://data.pdbj.org/pub/pdb/validation_reports/ay/8ayl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  15714MC  8aymC  8aynC  8ayoC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: ens_1
NCS oper: (Code: givenMatrix: (-0.999999534776, 0.000280959879172, 0.000922772238202), (-0.000281262083838, -0.999999906856, -0.000327383046606), (0.00092268017075, -0.000327642435143, 0.999999520656) ...NCS oper: (Code: given Matrix: (-0.999999534776, 0.000280959879172, 0.000922772238202), Vector: |
- Components
Components
-Isoform Flip of Glutamate receptor ... , 2 types, 4 molecules BDAC
| #1: Protein | Mass: 96247.055 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P19491 Homo sapiens (human) / References: UniProt: P19491#3: Protein | Mass: 102661.930 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P19490 Homo sapiens (human) / References: UniProt: P19490 |
|---|
-Protein , 1 types, 2 molecules JI
| #2: Protein | Mass: 43576.004 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: Q8VHW5 Homo sapiens (human) / References: UniProt: Q8VHW5 |
|---|
-Non-polymers , 5 types, 24 molecules 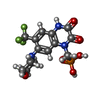



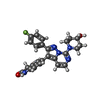




| #4: Chemical | ChemComp-ZK1 / {[ #5: Chemical | ChemComp-OLC / ( #6: Chemical | ChemComp-PLM / #7: Chemical | ChemComp-POV / ( #8: Chemical | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Resting state GluA1/A2 AMPA receptor in complex with TARP gamma 8 and ligand JNJ-61432059 Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 3000 nm / Nominal defocus min: 1200 nm |
| Specimen holder | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 315406 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 51.41 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||
| Refine LS restraints NCS | Type: NCS constraints / Rms dev position: 0.00069860284038 Å |
 Movie
Movie Controller
Controller





 PDBj
PDBj

















