[English] 日本語
 Yorodumi
Yorodumi- PDB-8anw: Poliovirus type 3 (strain Saukett) stabilised virus-like particle... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8anw | ||||||
|---|---|---|---|---|---|---|---|
| Title | Poliovirus type 3 (strain Saukett) stabilised virus-like particle (PV3 SC8). | ||||||
 Components Components |
| ||||||
 Keywords Keywords | VIRUS LIKE PARTICLE / Capsid protein | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome entry into host cell via pore formation in plasma membrane / viral capsid / host cell cytoplasm / symbiont-mediated suppression of host gene expression / virion attachment to host cell / structural molecule activity Similarity search - Function | ||||||
| Biological species |  Human poliovirus 3 Human poliovirus 3 | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.7 Å | ||||||
 Authors Authors | Bahar, M.W. / Fry, E.E. / Stuart, D.I. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Viruses / Year: 2022 Journal: Viruses / Year: 2022Title: Production and Characterisation of Stabilised PV-3 Virus-like Particles Using . Authors: Lee Sherry / Keith Grehan / Jessica J Swanson / Mohammad W Bahar / Claudine Porta / Elizabeth E Fry / David I Stuart / David J Rowlands / Nicola J Stonehouse /  Abstract: Following the success of global vaccination programmes using the live-attenuated oral and inactivated poliovirus vaccines (OPV and IPV), wild poliovirus (PV) is now only endemic in Afghanistan and ...Following the success of global vaccination programmes using the live-attenuated oral and inactivated poliovirus vaccines (OPV and IPV), wild poliovirus (PV) is now only endemic in Afghanistan and Pakistan. However, the continued use of these vaccines poses potential risks to the eradication of PV. The production of recombinant PV virus-like particles (VLPs), which lack the viral genome offer great potential as next-generation vaccines for the post-polio world. We have previously reported production of PV VLPs using , however, these VLPs were in the non-native conformation (C Ag), which would not produce effective protection against PV. Here, we build on this work and show that it is possible to produce wt PV-3 and thermally stabilised PV-3 (referred to as PV-3 SC8) VLPs in the native conformation (D Ag) using . We show that the PV-3 SC8 VLPs provide a much-improved D:C antigen ratio as compared to wt PV-3, whilst exhibiting greater thermostability than the current IPV vaccine. Finally, we determine the cryo-EM structure of the yeast-derived PV-3 SC8 VLPs and compare this to previously published PV-3 D Ag structures, highlighting the similarities between these recombinantly expressed VLPs and the infectious virus, further emphasising their potential as a next-generation vaccine candidate for PV. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8anw.cif.gz 8anw.cif.gz | 143.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8anw.ent.gz pdb8anw.ent.gz | 108 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8anw.json.gz 8anw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/an/8anw https://data.pdbj.org/pub/pdb/validation_reports/an/8anw ftp://data.pdbj.org/pub/pdb/validation_reports/an/8anw ftp://data.pdbj.org/pub/pdb/validation_reports/an/8anw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  15543MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 | x 60
|
- Components
Components
| #1: Protein | Mass: 33562.785 Da / Num. of mol.: 1 / Mutation: VP1 T105M, VP1 F132L Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Cell line (production host): PichiaPink / Production host: Human poliovirus 3 / Cell line (production host): PichiaPink / Production host:  Komagataella pastoris (fungus) / References: UniProt: Q84895 Komagataella pastoris (fungus) / References: UniProt: Q84895 |
|---|---|
| #2: Protein | Mass: 37623.023 Da / Num. of mol.: 1 / Mutation: VP2 L18I, VP2 L215M, VP2 D241E, VP4 T67A Source method: isolated from a genetically manipulated source Details: Sequence is given for the VP0 polypeptide. Mutations are numbered according to sequence numbering for mature polypeptides VP2 and VP4. Source: (gene. exp.)  Human poliovirus 3 / Cell line (production host): PichiaPink / Production host: Human poliovirus 3 / Cell line (production host): PichiaPink / Production host:  Komagataella pastoris (fungus) / References: UniProt: Q84895 Komagataella pastoris (fungus) / References: UniProt: Q84895 |
| #3: Protein | Mass: 26315.100 Da / Num. of mol.: 1 / Mutation: VP3 H19Y, VP3 L85F Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Cell line (production host): PichiaPink / Production host: Human poliovirus 3 / Cell line (production host): PichiaPink / Production host:  Komagataella pastoris (fungus) / References: UniProt: Q84895 Komagataella pastoris (fungus) / References: UniProt: Q84895 |
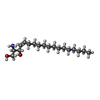
| #4: Chemical | ChemComp-SPH / |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human poliovirus 3 / Type: VIRUS Details: Recombinantly expressed virus-like particle of PV3 (strain Saukett). Entity ID: #1-#3 / Source: RECOMBINANT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 5.85 MDa / Experimental value: NO | ||||||||||||
| Source (natural) | Organism:  Human poliovirus 3 / Strain: Saukett Human poliovirus 3 / Strain: Saukett | ||||||||||||
| Source (recombinant) | Organism:  Komagataella pastoris (fungus) / Cell: PichiaPink Komagataella pastoris (fungus) / Cell: PichiaPink | ||||||||||||
| Details of virus | Empty: YES / Enveloped: NO / Isolate: SEROTYPE / Type: VIRUS-LIKE PARTICLE | ||||||||||||
| Natural host | Organism: Homo sapiens | ||||||||||||
| Virus shell | Name: Virus shell 1 / Diameter: 310 nm / Triangulation number (T number): 1 | ||||||||||||
| Buffer solution | pH: 7 / Details: 1 x DPBS, 20 mM EDTA, pH 7.0 | ||||||||||||
| Buffer component |
| ||||||||||||
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Virus-like particle purified by sucrose density gradient purification from Pichia pastoris cells. | ||||||||||||
| Specimen support | Details: The specific type of grid used was Ultra-thin carbon support film, 3nm - on lacey carbon AGS187-4 from Agar Scientific. Grid material: COPPER / Grid mesh size: 400 divisions/in. / Grid type: EMS Lacey Carbon | ||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE-PROPANE / Humidity: 100 % / Chamber temperature: 277.15 K Details: Double blotting with 4 ul of sample, followed by 4 second blot, before plunging. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 165000 X / Calibrated magnification: 60975 X / Nominal defocus max: 2400 nm / Nominal defocus min: 900 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 3 sec. / Electron dose: 35 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 3717 / Details: Pixel sampling of 0.82 A/pixel. |
| EM imaging optics | Energyfilter name: GIF Quantum LS / Energyfilter slit width: 20 eV |
| Image scans | Sampling size: 5 µm / Movie frames/image: 20 / Used frames/image: 1-20 |
- Processing
Processing
| Software | Name: PHENIX / Version: (1.19.2_4158:phenix.real_space_refine) / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Image processing | Details: Pixels size was 0.82 A/pixel | ||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 10744 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: I (icosahedral) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 2712 / Algorithm: BACK PROJECTION Details: Final reconstruction was sharpened with Post-processing in RELION using an inverse B-factor of -69.1 Angstroms. Num. of class averages: 2 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL / Target criteria: Correlation coefficient Details: Initial model was rigid body fitted using UCSF chimera and Coot. Global minimization and B-factor refinement was performed in real space using phenix_real.space.refine. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6Z6W Accession code: 6Z6W / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: THROUGHOUT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 57.31 Å2 / Biso mean: 13.0392 Å2 / Biso min: 5.18 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj