[English] 日本語
 Yorodumi
Yorodumi- PDB-8a9l: Cryo-EM structure of alpha-synuclein filaments from Parkinson's d... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8a9l | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of alpha-synuclein filaments from Parkinson's disease and dementia with Lewy bodies | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | PROTEIN FIBRIL / alpha-synuclein / amyloid / fibril / Parkinson's disease (PD) / Parkinson's disease dementia (PDD) / dementia with Lewy bodies (DLB) / synucleinopathy | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber ...negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / regulation of synaptic vesicle recycling / negative regulation of chaperone-mediated autophagy / mitochondrial membrane organization / regulation of reactive oxygen species biosynthetic process / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / SNARE complex assembly / regulation of locomotion / positive regulation of neurotransmitter secretion / negative regulation of dopamine metabolic process / positive regulation of inositol phosphate biosynthetic process / regulation of macrophage activation / regulation of norepinephrine uptake / negative regulation of microtubule polymerization / synaptic vesicle transport / transporter regulator activity / synaptic vesicle priming / dopamine uptake involved in synaptic transmission / protein kinase inhibitor activity / mitochondrial ATP synthesis coupled electron transport / regulation of dopamine secretion / dynein complex binding / negative regulation of thrombin-activated receptor signaling pathway / positive regulation of receptor recycling / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of endocytosis / positive regulation of exocytosis / synaptic vesicle exocytosis / kinesin binding / synaptic vesicle endocytosis / enzyme inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of serotonin uptake / response to type II interferon / regulation of presynapse assembly / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / supramolecular fiber organization / phospholipid metabolic process / cellular response to fibroblast growth factor stimulus / inclusion body / axon terminus / Hsp70 protein binding / cellular response to epinephrine stimulus / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / adult locomotory behavior / SNARE binding / excitatory postsynaptic potential / protein tetramerization / phosphoprotein binding / microglial cell activation / ferrous iron binding / fatty acid metabolic process / regulation of long-term neuronal synaptic plasticity / synapse organization / protein destabilization / PKR-mediated signaling / phospholipid binding / receptor internalization / tau protein binding / long-term synaptic potentiation / terminal bouton / positive regulation of inflammatory response / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress / neuron apoptotic process / cell cortex / response to lipopolysaccharide / histone binding / microtubule binding / chemical synaptic transmission / molecular adaptor activity / amyloid fibril formation / negative regulation of neuron apoptotic process / mitochondrial outer membrane / oxidoreductase activity Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | ELECTRON MICROSCOPY / helical reconstruction / cryo EM / Resolution: 2.2 Å | |||||||||||||||
 Authors Authors | Yang, Y. / Shi, Y. / Schweighauser, M. / Zhang, X.J. / Kotecha, A. / Murzin, A.G. / Garringer, H.J. / Cullinane, P. / Saito, Y. / Foroud, T. ...Yang, Y. / Shi, Y. / Schweighauser, M. / Zhang, X.J. / Kotecha, A. / Murzin, A.G. / Garringer, H.J. / Cullinane, P. / Saito, Y. / Foroud, T. / Warner, T.T. / Hasegawa, K. / Vidal, R. / Murayama, S. / Revesz, T. / Ghetti, B. / Hasegawa, M. / Lashley, T. / Scheres, H.W.S. / Goedert, M. | |||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structures of α-synuclein filaments from human brains with Lewy pathology. Authors: Yang Yang / Yang Shi / Manuel Schweighauser / Xianjun Zhang / Abhay Kotecha / Alexey G Murzin / Holly J Garringer / Patrick W Cullinane / Yuko Saito / Tatiana Foroud / Thomas T Warner / ...Authors: Yang Yang / Yang Shi / Manuel Schweighauser / Xianjun Zhang / Abhay Kotecha / Alexey G Murzin / Holly J Garringer / Patrick W Cullinane / Yuko Saito / Tatiana Foroud / Thomas T Warner / Kazuko Hasegawa / Ruben Vidal / Shigeo Murayama / Tamas Revesz / Bernardino Ghetti / Masato Hasegawa / Tammaryn Lashley / Sjors H W Scheres / Michel Goedert /     Abstract: Parkinson's disease (PD) is the most common movement disorder, with resting tremor, rigidity, bradykinesia and postural instability being major symptoms. Neuropathologically, it is characterized by ...Parkinson's disease (PD) is the most common movement disorder, with resting tremor, rigidity, bradykinesia and postural instability being major symptoms. Neuropathologically, it is characterized by the presence of abundant filamentous inclusions of α-synuclein in the form of Lewy bodies and Lewy neurites in some brain cells, including dopaminergic nerve cells of the substantia nigra. PD is increasingly recognised as a multisystem disorder, with cognitive decline being one of its most common non-motor symptoms. Many patients with PD develop dementia more than 10 years after diagnosis. PD dementia (PDD) is clinically and neuropathologically similar to dementia with Lewy bodies (DLB), which is diagnosed when cognitive impairment precedes parkinsonian motor signs or begins within one year from their onset. In PDD, cognitive impairment develops in the setting of well-established PD. Besides PD and DLB, multiple system atrophy (MSA) is the third major synucleinopathy. It is characterized by the presence of abundant filamentous α-synuclein inclusions in brain cells, especially oligodendrocytes (Papp-Lantos bodies). We previously reported the electron cryo-microscopy structures of two types of α-synuclein filament extracted from the brains of individuals with MSA. Each filament type is made of two different protofilaments. Here we report that the cryo-electron microscopy structures of α-synuclein filaments from the brains of individuals with PD, PDD and DLB are made of a single protofilament (Lewy fold) that is markedly different from the protofilaments of MSA. These findings establish the existence of distinct molecular conformers of assembled α-synuclein in neurodegenerative disease. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8a9l.cif.gz 8a9l.cif.gz | 29 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8a9l.ent.gz pdb8a9l.ent.gz | 15.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8a9l.json.gz 8a9l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8a9l_validation.pdf.gz 8a9l_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8a9l_full_validation.pdf.gz 8a9l_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  8a9l_validation.xml.gz 8a9l_validation.xml.gz | 20.3 KB | Display | |
| Data in CIF |  8a9l_validation.cif.gz 8a9l_validation.cif.gz | 27.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/a9/8a9l https://data.pdbj.org/pub/pdb/validation_reports/a9/8a9l ftp://data.pdbj.org/pub/pdb/validation_reports/a9/8a9l ftp://data.pdbj.org/pub/pdb/validation_reports/a9/8a9l | HTTPS FTP |
-Related structure data
| Related structure data |  15285MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 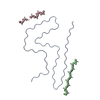
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 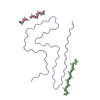
| ||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein | Mass: 14476.108 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: P37840 Homo sapiens (human) / References: UniProt: P37840 |
|---|---|
| #2: Protein/peptide | Mass: 783.958 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
| #3: Protein/peptide | Mass: 627.775 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method: helical reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Alpha-synuclein filaments extracted from the human brain with PD, PDD, and DLB Type: TISSUE / Entity ID: #1-#3 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1400 nm / Nominal defocus min: 600 nm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.8.0350 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Helical symmerty | Angular rotation/subunit: 0.86 ° / Axial rise/subunit: 4.76 Å / Axial symmetry: C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 68330 / Symmetry type: HELICAL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 2.2→2.2 Å / Cor.coef. Fo:Fc: 0.769 / SU B: 5.034 / SU ML: 0.126 / ESU R: 0.09 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 51.001 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 576 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj
