[English] 日本語
 Yorodumi
Yorodumi- PDB-7yu5: Human Lysophosphatidic Acid Receptor 1-Gi complex bound to ONO-07... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7yu5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human Lysophosphatidic Acid Receptor 1-Gi complex bound to ONO-0740556, state1 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / GPCR | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to 1-oleoyl-sn-glycerol 3-phosphate / lysophosphatidic acid receptor activity / positive regulation of smooth muscle cell chemotaxis / Lysosphingolipid and LPA receptors / lysophosphatidic acid binding / negative regulation of cilium assembly / corpus callosum development / bleb assembly / optic nerve development / cellular response to oxygen levels ...cellular response to 1-oleoyl-sn-glycerol 3-phosphate / lysophosphatidic acid receptor activity / positive regulation of smooth muscle cell chemotaxis / Lysosphingolipid and LPA receptors / lysophosphatidic acid binding / negative regulation of cilium assembly / corpus callosum development / bleb assembly / optic nerve development / cellular response to oxygen levels / oligodendrocyte development / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Ca2+ pathway / regulation of synaptic vesicle cycle / G alpha (z) signalling events / negative regulation of cAMP/PKA signal transduction / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / regulation of metabolic process / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / photoreceptor outer segment membrane / G alpha (q) signalling events / spectrin binding / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / regulation of postsynaptic neurotransmitter receptor internalization / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of Rho protein signal transduction / positive regulation of dendritic spine development / alkylglycerophosphoethanolamine phosphodiesterase activity / G-protein alpha-subunit binding / photoreceptor outer segment / adenylate cyclase inhibitor activity / positive regulation of stress fiber assembly / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / cardiac muscle cell apoptotic process / neurogenesis / myelination / photoreceptor inner segment / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / cerebellum development / regulation of mitotic spindle organization / dendritic shaft / cell chemotaxis / PDZ domain binding / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / G protein-coupled receptor activity / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / GABA-ergic synapse / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway Similarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||
 Authors Authors | Akasaka, H. / Shihoya, W. / Nureki, O. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structure of the active G-coupled human lysophosphatidic acid receptor 1 complexed with a potent agonist. Authors: Hiroaki Akasaka / Tatsuki Tanaka / Fumiya K Sano / Yuma Matsuzaki / Wataru Shihoya / Osamu Nureki /  Abstract: Lysophosphatidic acid receptor 1 (LPA) is one of the six G protein-coupled receptors activated by the bioactive lipid, lysophosphatidic acid (LPA). LPA is a drug target for various diseases, ...Lysophosphatidic acid receptor 1 (LPA) is one of the six G protein-coupled receptors activated by the bioactive lipid, lysophosphatidic acid (LPA). LPA is a drug target for various diseases, including cancer, inflammation, and neuropathic pain. Notably, LPA agonists have potential therapeutic value for obesity and urinary incontinence. Here, we report a cryo-electron microscopy structure of the active human LPA-G complex bound to ONO-0740556, an LPA analog with more potent activity against LPA. Our structure elucidated the details of the agonist binding mode and receptor activation mechanism mediated by rearrangements of transmembrane segment 7 and the central hydrophobic core. A structural comparison of LPA and other phylogenetically-related lipid-sensing GPCRs identified the structural determinants for lipid preference of LPA. Moreover, we characterized the structural polymorphisms at the receptor-G-protein interface, which potentially reflect the G-protein dissociation process. Our study provides insights into the detailed mechanism of LPA binding to agonists and paves the way toward the design of drug-like agonists targeting LPA. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7yu5.cif.gz 7yu5.cif.gz | 242.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7yu5.ent.gz pdb7yu5.ent.gz | 184.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7yu5.json.gz 7yu5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7yu5_validation.pdf.gz 7yu5_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7yu5_full_validation.pdf.gz 7yu5_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  7yu5_validation.xml.gz 7yu5_validation.xml.gz | 34.4 KB | Display | |
| Data in CIF |  7yu5_validation.cif.gz 7yu5_validation.cif.gz | 54.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yu/7yu5 https://data.pdbj.org/pub/pdb/validation_reports/yu/7yu5 ftp://data.pdbj.org/pub/pdb/validation_reports/yu/7yu5 ftp://data.pdbj.org/pub/pdb/validation_reports/yu/7yu5 | HTTPS FTP |
-Related structure data
| Related structure data |  34099MC  7yu3C  7yu4C  7yu6C  7yu7C  7yu8C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules BAG
| #1: Protein | Mass: 38744.371 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Baculovirus expression vector pFastBac1-HM / References: UniProt: P54311 Baculovirus expression vector pFastBac1-HM / References: UniProt: P54311 |
|---|---|
| #2: Protein | Mass: 40415.031 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAI1 / Production host: Homo sapiens (human) / Gene: GNAI1 / Production host:  Baculovirus expression vector pFastBac1-HM / References: UniProt: P63096 Baculovirus expression vector pFastBac1-HM / References: UniProt: P63096 |
| #3: Protein | Mass: 7547.685 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Baculovirus expression vector pFastBac1-HM / References: UniProt: P63212 Baculovirus expression vector pFastBac1-HM / References: UniProt: P63212 |
-Protein / Antibody / Non-polymers , 3 types, 3 molecules RS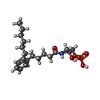

| #4: Protein | Mass: 42936.004 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: LPAR1, EDG2, LPA1 / Production host: Homo sapiens (human) / Gene: LPAR1, EDG2, LPA1 / Production host:  Baculovirus expression vector pFastBac1-HM / References: UniProt: Q92633 Baculovirus expression vector pFastBac1-HM / References: UniProt: Q92633 |
|---|---|
| #5: Antibody | Mass: 27720.795 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| #6: Chemical | ChemComp-K6L / [( |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight |
| ||||||||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 7 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.8.0267 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 129275 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 3.7→3.7 Å / Cor.coef. Fo:Fc: 0.928 / SU B: 19.798 / SU ML: 0.302 / ESU R: 0.377 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 138.564 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 8788 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj






















