+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7wzv | ||||||
|---|---|---|---|---|---|---|---|
| Title | The structure of a Twitch Radical SAM Dehydrogenase SpeY | ||||||
 Components Components | 4Fe-4S cluster-binding domain-containing protein | ||||||
 Keywords Keywords | BIOSYNTHETIC PROTEIN / Twitch Radical SAM Dehydrogenase / Spectinomycin / X-ray crystallography | ||||||
| Function / homology |  Function and homology information Function and homology informationcatalytic activity / 4 iron, 4 sulfur cluster binding / metal ion binding Similarity search - Function | ||||||
| Biological species |  Streptomyces spectabilis (bacteria) Streptomyces spectabilis (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.89931293521 Å SAD / Resolution: 1.89931293521 Å | ||||||
 Authors Authors | Zhou, J.H. / Hou, X.L. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: J.Am.Chem.Soc. / Year: 2022 Journal: J.Am.Chem.Soc. / Year: 2022Title: Dioxane Bridge Formation during the Biosynthesis of Spectinomycin Involves a Twitch Radical S -Adenosyl Methionine Dehydrogenase That May Have Evolved from an Epimerase. Authors: Zhang, J. / Hou, X. / Chen, Z. / Ko, Y. / Ruszczycky, M.W. / Chen, Y. / Zhou, J. / Liu, H.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7wzv.cif.gz 7wzv.cif.gz | 320.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7wzv.ent.gz pdb7wzv.ent.gz | 213.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7wzv.json.gz 7wzv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wz/7wzv https://data.pdbj.org/pub/pdb/validation_reports/wz/7wzv ftp://data.pdbj.org/pub/pdb/validation_reports/wz/7wzv ftp://data.pdbj.org/pub/pdb/validation_reports/wz/7wzv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7wzxC  7x0bC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Ens-ID: 1 / Beg auth comp-ID: VAL / Beg label comp-ID: VAL / End auth comp-ID: VAL / End label comp-ID: VAL / Auth seq-ID: 3 - 299 / Label seq-ID: 23 - 319
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 36014.637 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Streptomyces spectabilis (bacteria) / Gene: speY, FH965_34950 / Production host: Streptomyces spectabilis (bacteria) / Gene: speY, FH965_34950 / Production host:  |
|---|
-Non-polymers , 9 types, 390 molecules 

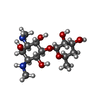














| #2: Chemical | ChemComp-SF4 / #3: Chemical | #4: Chemical | #5: Chemical | ChemComp-SO4 / #6: Chemical | ChemComp-NA / #7: Chemical | ChemComp-GOL / #8: Chemical | ChemComp-CL / | #9: Chemical | ChemComp-EDO / | #10: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.45 Å3/Da / Density % sol: 49.8 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 0.5 M Sodium cacodylate trihydrate (pH 6.5), 1.7 M Ammonium sulfate and 0.05 M Magnesium acetate tetrahydrate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U1 / Wavelength: 0.97917 Å / Beamline: BL17U1 / Wavelength: 0.97917 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Jul 18, 2020 |
| Radiation | Monochromator: Si111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97917 Å / Relative weight: 1 |
| Reflection | Resolution: 1.89→70.77 Å / Num. obs: 53042 / % possible obs: 100 % / Redundancy: 13 % / CC1/2: 0.999 / Net I/σ(I): 16.7 |
| Reflection shell | Resolution: 1.89→2 Å / Num. unique obs: 7642 / CC1/2: 0.79 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.89931293521→70.7643857242 Å / SU ML: 0.240473563064 / Cross valid method: THROUGHOUT / σ(F): 1.32609457704 / Phase error: 25.1162430572 SAD / Resolution: 1.89931293521→70.7643857242 Å / SU ML: 0.240473563064 / Cross valid method: THROUGHOUT / σ(F): 1.32609457704 / Phase error: 25.1162430572 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.2001153623 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.89931293521→70.7643857242 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -4.07118898352 Å / Origin y: 16.4696925026 Å / Origin z: -27.1929538399 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller



 PDBj
PDBj








