[English] 日本語
 Yorodumi
Yorodumi- PDB-7vgu: Time-resolved serial femtosecond crystallography structure of lig... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7vgu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Time-resolved serial femtosecond crystallography structure of light-driven chloride ion-pumping rhodopsin, NM-R3 : structure obtained 1 msec after photoexcitation with bromide ion | ||||||
 Components Components | Chloride pumping rhodopsin | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / SACLA serial femtosecond crystallography CELL-FREE SYNTHESIS Bacterial type rhodopsin chloride ion pump rhodopsin | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Nonlabens marinus S1-08 (bacteria) Nonlabens marinus S1-08 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / FREE ELECTRON LASER /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Hosaka, T. / Nango, E. / Nakane, T. / Luo, F. / Kimura-Someya, T. / Shirouzu, M. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2022 Journal: Proc.Natl.Acad.Sci.USA / Year: 2022Title: Conformational alterations in unidirectional ion transport of a light-driven chloride pump revealed using X-ray free electron lasers. Authors: Hosaka, T. / Nomura, T. / Kubo, M. / Nakane, T. / Fangjia, L. / Sekine, S.I. / Ito, T. / Murayama, K. / Ihara, K. / Ehara, H. / Kashiwagi, K. / Katsura, K. / Akasaka, R. / Hisano, T. / ...Authors: Hosaka, T. / Nomura, T. / Kubo, M. / Nakane, T. / Fangjia, L. / Sekine, S.I. / Ito, T. / Murayama, K. / Ihara, K. / Ehara, H. / Kashiwagi, K. / Katsura, K. / Akasaka, R. / Hisano, T. / Tanaka, T. / Tanaka, R. / Arima, T. / Yamashita, A. / Sugahara, M. / Naitow, H. / Matsuura, Y. / Yoshizawa, S. / Tono, K. / Owada, S. / Nureki, O. / Kimura-Someya, T. / Iwata, S. / Nango, E. / Shirouzu, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7vgu.cif.gz 7vgu.cif.gz | 76.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7vgu.ent.gz pdb7vgu.ent.gz | 55 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7vgu.json.gz 7vgu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7vgu_validation.pdf.gz 7vgu_validation.pdf.gz | 3 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7vgu_full_validation.pdf.gz 7vgu_full_validation.pdf.gz | 3 MB | Display | |
| Data in XML |  7vgu_validation.xml.gz 7vgu_validation.xml.gz | 13.1 KB | Display | |
| Data in CIF |  7vgu_validation.cif.gz 7vgu_validation.cif.gz | 17.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vg/7vgu https://data.pdbj.org/pub/pdb/validation_reports/vg/7vgu ftp://data.pdbj.org/pub/pdb/validation_reports/vg/7vgu ftp://data.pdbj.org/pub/pdb/validation_reports/vg/7vgu | HTTPS FTP |
-Related structure data
| Related structure data |  7vgtC  7vgvC  5b2nS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | |
| Experimental dataset #1 | Data reference:  10.11577/1843782 / Data set type: diffraction image data 10.11577/1843782 / Data set type: diffraction image data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 30710.936 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Nonlabens marinus S1-08 (bacteria) / Gene: ClR, NMS_1267 / Production host: Nonlabens marinus S1-08 (bacteria) / Gene: ClR, NMS_1267 / Production host:  |
|---|
-Non-polymers , 5 types, 66 molecules 
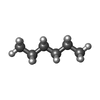







| #2: Chemical | ChemComp-RET / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-HEX / #4: Chemical | #5: Chemical | #6: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.55 Å3/Da / Density % sol: 51.72 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: lipidic cubic phase / pH: 7 Details: 38% PEG400, 300 mM K phosphate, and 100 mM HEPES (pH7.0) |
-Data collection
| Diffraction | Mean temperature: 293 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  SACLA SACLA  / Beamline: BL3 / Wavelength: 1.7714 Å / Beamline: BL3 / Wavelength: 1.7714 Å |
| Detector | Type: MPCCD / Detector: CCD / Date: Feb 2, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.7714 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→42.8 Å / Num. obs: 18261 / % possible obs: 100 % / Redundancy: 363 % / CC1/2: 0.993 / Net I/σ(I): 11.6 |
| Reflection shell | Resolution: 2.1→2.14 Å / Num. unique obs: 929 / CC1/2: 0.918 |
| Serial crystallography sample delivery | Method: injection |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5B2N Resolution: 2.1→29.36 Å / SU ML: 0.18 / Cross valid method: THROUGHOUT / σ(F): 1.37 / Phase error: 21.42 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 149.38 Å2 / Biso mean: 32.8699 Å2 / Biso min: 14.98 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.1→29.36 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 6
|
 Movie
Movie Controller
Controller












 PDBj
PDBj











