[English] 日本語
 Yorodumi
Yorodumi- PDB-7upf: Tau Paired Helical Filament from Alzheimer's Disease incubated 1 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7upf | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tau Paired Helical Filament from Alzheimer's Disease incubated 1 hr. with EGCG | |||||||||||||||||||||||||||||||||
 Components Components | Isoform Tau-F of Microtubule-associated protein tau | |||||||||||||||||||||||||||||||||
 Keywords Keywords | PROTEIN FIBRIL / Amyloid / fibril / Alzheimer's | |||||||||||||||||||||||||||||||||
| Function / homology | Activation of AMPK downstream of NMDARs / PKR-mediated signaling / Isoform Tau-F of Microtubule-associated protein tau Function and homology information Function and homology information | |||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / helical reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||||||||||||||||||||||||||
 Authors Authors | Seidler, P.M. / Murray, K.A. / Boyer, D.R. / Ge, P. / Sawaya, M.R. / Eisenberg, D.S. | |||||||||||||||||||||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structure-based discovery of small molecules that disaggregate Alzheimer's disease tissue derived tau fibrils in vitro. Authors: Paul M Seidler / Kevin A Murray / David R Boyer / Peng Ge / Michael R Sawaya / Carolyn J Hu / Xinyi Cheng / Romany Abskharon / Hope Pan / Michael A DeTure / Christopher K Williams / Dennis W ...Authors: Paul M Seidler / Kevin A Murray / David R Boyer / Peng Ge / Michael R Sawaya / Carolyn J Hu / Xinyi Cheng / Romany Abskharon / Hope Pan / Michael A DeTure / Christopher K Williams / Dennis W Dickson / Harry V Vinters / David S Eisenberg /  Abstract: Alzheimer's disease (AD) is the consequence of neuronal death and brain atrophy associated with the aggregation of protein tau into fibrils. Thus disaggregation of tau fibrils could be a therapeutic ...Alzheimer's disease (AD) is the consequence of neuronal death and brain atrophy associated with the aggregation of protein tau into fibrils. Thus disaggregation of tau fibrils could be a therapeutic approach to AD. The small molecule EGCG, abundant in green tea, has long been known to disaggregate tau and other amyloid fibrils, but EGCG has poor drug-like properties, failing to fully penetrate the brain. Here we have cryogenically trapped an intermediate of brain-extracted tau fibrils on the kinetic pathway to EGCG-induced disaggregation and have determined its cryoEM structure. The structure reveals that EGCG molecules stack in polar clefts between the paired helical protofilaments that pathologically define AD. Treating the EGCG binding position as a pharmacophore, we computationally screened thousands of drug-like compounds for compatibility for the pharmacophore, discovering several that experimentally disaggregate brain-derived tau fibrils in vitro. This work suggests the potential of structure-based, small-molecule drug discovery for amyloid diseases. | |||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7upf.cif.gz 7upf.cif.gz | 177.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7upf.ent.gz pdb7upf.ent.gz | 117.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7upf.json.gz 7upf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7upf_validation.pdf.gz 7upf_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7upf_full_validation.pdf.gz 7upf_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  7upf_validation.xml.gz 7upf_validation.xml.gz | 29.8 KB | Display | |
| Data in CIF |  7upf_validation.cif.gz 7upf_validation.cif.gz | 42.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/up/7upf https://data.pdbj.org/pub/pdb/validation_reports/up/7upf ftp://data.pdbj.org/pub/pdb/validation_reports/up/7upf ftp://data.pdbj.org/pub/pdb/validation_reports/up/7upf | HTTPS FTP |
-Related structure data
| Related structure data |  26664MC  7upeC  7upgC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 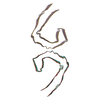
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 45919.871 Da / Num. of mol.: 10 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MAPT, MAPTL, MTBT1, TAU / Production host: Homo sapiens (human) / Gene: MAPT, MAPTL, MTBT1, TAU / Production host:  Homo sapiens (human) / References: UniProt: P10636-8 Homo sapiens (human) / References: UniProt: P10636-8Has protein modification | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: HELICAL ARRAY / 3D reconstruction method: helical reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Tau Alzheimer's Disease Paired Helical Filament incubated 1 hr. with EGCG Type: TISSUE / Entity ID: all / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 1.21 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.2_4158: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Helical symmerty | Angular rotation/subunit: 179.47 ° / Axial rise/subunit: 2.4 Å / Axial symmetry: C1 | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 20759 / Symmetry type: HELICAL | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj



