[English] 日本語
 Yorodumi
Yorodumi- PDB-7q0s: Human GYS1-GYG1 complex inhibited-like state bound to glucose-6-p... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7q0s | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human GYS1-GYG1 complex inhibited-like state bound to glucose-6-phosphate | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE / Glycogen / Complex / Phosphorylation | ||||||
| Function / homology |  Function and homology information Function and homology informationglycogen synthase activity, transferring glucose-1-phosphate / Glycogen storage disease type XV (GYG1) / Glycogen storage disease type 0 (muscle GYS1) / glycogen(starch) synthase / glycogenin glucosyltransferase / Glycogen storage disease type II (GAA) / glycogenin glucosyltransferase activity / alpha-1,4-glucan glucosyltransferase (UDP-glucose donor) activity / D-glucose binding / glycogen biosynthetic process ...glycogen synthase activity, transferring glucose-1-phosphate / Glycogen storage disease type XV (GYG1) / Glycogen storage disease type 0 (muscle GYS1) / glycogen(starch) synthase / glycogenin glucosyltransferase / Glycogen storage disease type II (GAA) / glycogenin glucosyltransferase activity / alpha-1,4-glucan glucosyltransferase (UDP-glucose donor) activity / D-glucose binding / glycogen biosynthetic process / Glycogen breakdown (glycogenolysis) / glycosyltransferase activity / inclusion body / Myoclonic epilepsy of Lafora / Glycogen synthesis / lysosomal lumen / manganese ion binding / heart development / secretory granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation / protein homodimerization activity / extracellular region / nucleus / membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4 Å | ||||||
 Authors Authors | McCorvie, T.J. / Shrestha, L. / Froese, D.S. / Ferreira, I.M. / Yue, W.W. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Molecular basis for the regulation of human glycogen synthase by phosphorylation and glucose-6-phosphate. Authors: Thomas J McCorvie / Paula M Loria / Meihua Tu / Seungil Han / Leela Shrestha / D Sean Froese / Igor M Ferreira / Allison P Berg / Wyatt W Yue /    Abstract: Glycogen synthase (GYS1) is the central enzyme in muscle glycogen biosynthesis. GYS1 activity is inhibited by phosphorylation of its amino (N) and carboxyl (C) termini, which is relieved by ...Glycogen synthase (GYS1) is the central enzyme in muscle glycogen biosynthesis. GYS1 activity is inhibited by phosphorylation of its amino (N) and carboxyl (C) termini, which is relieved by allosteric activation of glucose-6-phosphate (Glc6P). We present cryo-EM structures at 3.0-4.0 Å resolution of phosphorylated human GYS1, in complex with a minimal interacting region of glycogenin, in the inhibited, activated and catalytically competent states. Phosphorylations of specific terminal residues are sensed by different arginine clusters, locking the GYS1 tetramer in an inhibited state via intersubunit interactions. The Glc6P activator promotes conformational change by disrupting these interactions and increases the flexibility of GYS1, such that it is poised to adopt a catalytically competent state when the sugar donor UDP-glucose (UDP-glc) binds. We also identify an inhibited-like conformation that has not transitioned into the activated state, in which the locking interaction of phosphorylation with the arginine cluster impedes subsequent conformational changes due to Glc6P binding. Our results address longstanding questions regarding the mechanism of human GYS1 regulation. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7q0s.cif.gz 7q0s.cif.gz | 479.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7q0s.ent.gz pdb7q0s.ent.gz | 385.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7q0s.json.gz 7q0s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q0/7q0s https://data.pdbj.org/pub/pdb/validation_reports/q0/7q0s ftp://data.pdbj.org/pub/pdb/validation_reports/q0/7q0s ftp://data.pdbj.org/pub/pdb/validation_reports/q0/7q0s | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  13751MC  7q0bC  7q12C  7q13C C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
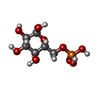
| #1: Protein | Mass: 83885.430 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GYS1, GYS / Organ: Muscel / Production host: Homo sapiens (human) / Gene: GYS1, GYS / Organ: Muscel / Production host:  #2: Protein | Mass: 83965.406 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Tissue: Muscle / Gene: GYS1, GYS / Production host: Homo sapiens (human) / Tissue: Muscle / Gene: GYS1, GYS / Production host:  #3: Protein | Mass: 39422.621 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GYG1, GYG / Production host: Homo sapiens (human) / Gene: GYG1, GYG / Production host:  #4: Sugar | ChemComp-G6P / Has ligand of interest | Y | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Ternary complex of phosphorylated full-length human glycogen synthase 1 in complex with minimum region of human glycogenin-1 Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.35 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) / Tissue: muscle Homo sapiens (human) / Tissue: muscle | ||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 Details: 25 mM HEPES, pH 7.5, 200 mM NaCl, 2.0 mM TCEP, 0.05% (v/v) tween-20 filtered sterilised with 5 mM glucose-6-phosphate | ||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.75 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK II / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 81000 X / Nominal defocus max: 2300 nm / Nominal defocus min: 800 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 1.22 sec. / Electron dose: 55 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.2_4158: / Classification: refinement | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 4391867 | ||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: D2 (2x2 fold dihedral) | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 40062 / Algorithm: FOURIER SPACE / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 81.36 / Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: correlation coefficient | ||||||||||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Accession code: 4QLB / Initial refinement model-ID: 1 / PDB-ID: 4QLB / Source name: PDB / Type: experimental model
|
 Movie
Movie Controller
Controller





 PDBj
PDBj