+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7oyk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
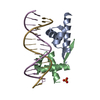
| Title | DNA-binding domain of CggR in complex with the DNA operator | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | DNA BINDING PROTEIN / Transcriptional repressor / Glycolysis / Helix-turn-helix domain / Bacillus subtilis | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of DNA-templated transcription initiation / cis-regulatory region sequence-specific DNA binding / carbohydrate binding Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.101 Å SAD / Resolution: 2.101 Å | |||||||||
 Authors Authors | Novakova, M. / Rezacova, P. / Skerlova, J. / Brynda, J. | |||||||||
| Funding support |  Czech Republic, 2items Czech Republic, 2items
| |||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2021 Journal: Acta Crystallogr D Struct Biol / Year: 2021Title: Structural insight into DNA recognition by bacterial transcriptional regulators of the SorC/DeoR family. Authors: Soltysova, M. / Sieglova, I. / Fabry, M. / Brynda, J. / Skerlova, J. / Rezacova, P. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7oyk.cif.gz 7oyk.cif.gz | 146.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7oyk.ent.gz pdb7oyk.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7oyk.json.gz 7oyk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oy/7oyk https://data.pdbj.org/pub/pdb/validation_reports/oy/7oyk ftp://data.pdbj.org/pub/pdb/validation_reports/oy/7oyk ftp://data.pdbj.org/pub/pdb/validation_reports/oy/7oyk | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 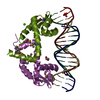
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules AAADDDCCCBBB
| #1: Protein | Mass: 11121.280 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: The first amino-acid residues "SNAAS" are remnants after the cleavage of the His-tag by TEV protease. Source: (gene. exp.)  Gene: cggR, yvbQ, BSU33950 / Plasmid: pET151/D-TOPO / Production host:  |
|---|
-DNA operator - strand ... , 2 types, 6 molecules EEELLLGGGFFFKKKHHH
| #2: DNA chain | Mass: 4885.209 Da / Num. of mol.: 3 / Source method: obtained synthetically Source: (synth.)  #3: DNA chain | Mass: 4911.173 Da / Num. of mol.: 3 / Source method: obtained synthetically Source: (synth.)  |
|---|
-Non-polymers , 4 types, 144 molecules 






| #4: Chemical | | #5: Chemical | ChemComp-EDO / | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.07 Å3/Da / Density % sol: 40.53 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 10% (w/v) PEG 3350, 100mM MES, pH 6.5, 100mM calcium chloride, 13% (v/v) glycerol |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.2 / Wavelength: 0.9797 Å / Beamline: 14.2 / Wavelength: 0.9797 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jun 3, 2020 |
| Radiation | Monochromator: DCM Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9797 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→50 Å / Num. obs: 34417 / % possible obs: 96.8 % / Redundancy: 2.3 % / Biso Wilson estimate: 46.7 Å2 / CC1/2: 0.998 / Rrim(I) all: 0.086 / Net I/σ(I): 9.4 |
| Reflection shell | Resolution: 2.1→2.23 Å / Redundancy: 2.3 % / Mean I/σ(I) obs: 1.1 / Num. unique obs: 10673 / CC1/2: 0.514 / Rrim(I) all: 1.111 / % possible all: 95.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 2.101→45.837 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.937 / WRfactor Rfree: 0.222 / WRfactor Rwork: 0.183 / Average fsc free: 0.844 / Average fsc work: 0.8563 / Cross valid method: THROUGHOUT / ESU R: 0.294 / ESU R Free: 0.216 SAD / Resolution: 2.101→45.837 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.937 / WRfactor Rfree: 0.222 / WRfactor Rwork: 0.183 / Average fsc free: 0.844 / Average fsc work: 0.8563 / Cross valid method: THROUGHOUT / ESU R: 0.294 / ESU R Free: 0.216 Details: Hydrogens have been added in their riding positions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK BULK SOLVENT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 43.543 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.101→45.837 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj









































